


| |
 Crystal structure | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
PubChem CID |
|
| |
| |
| Properties | |
| Cl3Cm | |
| Molar mass | 353 g·mol−1 |
| Appearance | White solid (anhydrous) Light green solid (hydrate) |
| Melting point | 695 °C (1,283 °F; 968 K)[citation needed] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Curium(III) chloride is the chemical compound with the formula CmCl3.
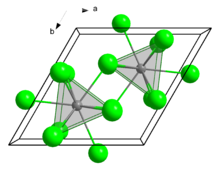
Curium(III) chloride has a 9 coordinate tricapped trigonal prismatic geometry.[1]
Curium(III) chloride can be obtained from the reaction of hydrogen chloride gas with curium dioxide, curium(III) oxide, or curium(III) oxychloride at a temperature of 400-600 °C:
It can also be obtained from the dissolution of metallic curium in dilute hydrochloric acid:[2]
This method has a number of disadvantages associated with the ongoing processes of hydrolysis and hydration of the resulting compound in an aqueous solution, making it problematic to obtain a pure product using this reaction.
It can be obtained from the reaction of curium nitride with cadmium chloride:[3]
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
This nuclear technology article is a stub. You can help Wikipedia by expanding it. |
This inorganic compound–related article is a stub. You can help Wikipedia by expanding it. |