


| |

| |
| Names | |
|---|---|
| IUPAC name
Platinum tetrachloride | |
| Other names
Platinum(IV) chloride | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.033.300 |
| EC Number |
|
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| PtCl4 | |
| Molar mass | 336.89 g/mol |
| Appearance | brown-red powder |
| Density | 4.303 g/cm3 (anhydrous) 2.43 g/cm3 (pentahydrate) |
| Melting point | 370 °C (698 °F; 643 K) (decomposes) |
| 58.7 g/100 mL (anhydrous) very soluble (pentahydrate) | |
| Solubility | anhydrous soluble in acetone slightly soluble in ethanol insoluble in ether pentahydrate soluble in alcohol, ether |
| −93.0·10−6cm3/mol | |
| Structure | |
| Square planar | |
| Hazards | |
| GHS labelling:[1] | |
   
| |
| Danger | |
| H290, H301, H314, H317, H334 | |
| P234, P260, P261, P264, P270, P272, P280, P285, P301+P310, P301+P330+P331, P302+P352, P303+P361+P353, P304+P340, P304+P341, P305+P351+P338, P310, P321, P330, P333+P313, P342+P311, P363, P390, P404, P405, P501 | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
276 mg/kg (rat, oral) |
| Related compounds | |
Other anions |
Platinum(IV) bromide Platinum(IV) fluoride Platinum(IV) sulfide |
Other cations |
Iridium(IV) chloride |
Related compounds |
Platinum(II) chloride Platinum(VI) fluoride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Platinum(IV) chloride is the inorganic compoundofplatinum and chlorine with the empirical formula PtCl4. This brown solid features platinum in the 4+ oxidation state.
Typical of Pt(IV), the metal centers adopt an octahedral coordination geometry, {PtCl6}. This geometry is achieved by forming a polymer wherein half of the chloride ligands bridge between the platinum centers. Because of its polymeric structure, PtCl4 dissolves only upon breaking the chloride bridging ligands. Thus, addition of HCl give H2PtCl6. Lewis base adducts of Pt(IV) of the type cis-PtCl4L2 are known, but most are prepared by oxidation of the Pt(II) derivatives.[2]
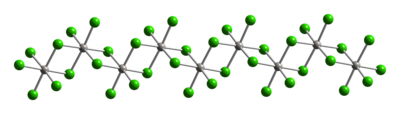
|
| Part of a (PtCl4)∞ chain from the crystal structure of platinum(IV) chloride |
PtCl4 is mainly encountered in the handling of chloroplatinic acid, obtained by dissolving of Pt metal in aqua regia. Heating H2PtCl6 to 220 °C gives impure PtCl4:[3]
A purer product can be produced by heating under chlorine gas at 250 °C.[4]
If excess acids are removed, PtCl4 crystallizes from aqueous solutions in large red crystals of pentahydrate PtCl4·5(H2O),[5] which can be dehydrated by heating to about 300 °C in a current of dry chlorine. The pentahydrate is stable and is used as the commercial form of PtCl4.
Treatment of PtCl4 with aqueous base gives the [Pt(OH)6]2− ion. With methyl Grignard reagents followed by partial hydrolysis, PtCl4 converts to the cuboidal cluster [Pt(CH3)3(OH)]4.[6] Upon heating PtCl4 evolves chlorine to give PtCl2:
The heavier halides, PtBr4 and PtI4, are also known.
|
| |||
|---|---|---|---|
| Pt(-II) |
| ||
| Pt(0) |
| ||
| Pt(II) |
| ||
| Pt(IV) |
| ||
| Pt(V) |
| ||
| Pt(VI) |
| ||