J u m p t o c o n t e n t
M a i n m e n u
M a i n m e n u
N a v i g a t i o n
● M a i n p a g e ● C o n t e n t s ● C u r r e n t e v e n t s ● R a n d o m a r t i c l e ● A b o u t W i k i p e d i a ● C o n t a c t u s ● D o n a t e
C o n t r i b u t e
● H e l p ● L e a r n t o e d i t ● C o m m u n i t y p o r t a l ● R e c e n t c h a n g e s ● U p l o a d f i l e
S e a r c h
Search
A p p e a r a n c e
● C r e a t e a c c o u n t ● L o g i n
P e r s o n a l t o o l s
● C r e a t e a c c o u n t ● L o g i n
P a g e s f o r l o g g e d o u t e d i t o r s l e a r n m o r e ● C o n t r i b u t i o n s ● T a l k
( T o p )
1 P r e p a r a t i o n , s t r u c t u r e , r e a c t i o n s
2 R e f e r e n c e s
T o g g l e t h e t a b l e o f c o n t e n t s
O s m i u m ( IV ) c h l o r i d e
1 6 l a n g u a g e s
● ت ۆ ر ک ج ه ● Č e š t i n a ● D e u t s c h ● ف ا ر س ی ● F r a n ç a i s ● I t a l i a n o ● N e d e r l a n d s ● 日 本 語 ● P o r t u g u ê s ● Р у с с к и й ● S l o v e n č i n a ● С р п с к и / s r p s k i ● S r p s k o h r v a t s k i / с р п с к о х р в а т с к и ● S u o m i ● த ம ி ழ ் ● 中 文
E d i t l i n k s
● A r t i c l e ● T a l k
E n g l i s h
● R e a d ● E d i t ● V i e w h i s t o r y
T o o l s
T o o l s
A c t i o n s
● R e a d ● E d i t ● V i e w h i s t o r y
G e n e r a l
● W h a t l i n k s h e r e ● R e l a t e d c h a n g e s ● U p l o a d f i l e ● S p e c i a l p a g e s ● P e r m a n e n t l i n k ● P a g e i n f o r m a t i o n ● C i t e t h i s p a g e ● G e t s h o r t e n e d U R L ● D o w n l o a d Q R c o d e ● W i k i d a t a i t e m
P r i n t / e x p o r t
● D o w n l o a d a s P D F ● P r i n t a b l e v e r s i o n
I n o t h e r p r o j e c t s
● W i k i m e d i a C o m m o n s
A p p e a r a n c e
F r o m W i k i p e d i a , t h e f r e e e n c y c l o p e d i a
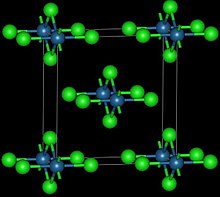
Osmium(IV ) chloride or osmium tetrachloride is the inorganic compound composed of osmium and chlorine with the empirical formula OsCl4 polymorphs (crystalline forms). The compound is used to prepare other osmium complexes.
[ edit ]
It was first reported in 1909 as the product of chlorination of osmium metal.[1] [2]
Os + 2 Cl2 4
This reddish-black polymorph is orthorhombic and adopts a structure in which osmium centres are octahedrally coordinated, sharing opposite edges of the OsCl6 [3] osmium tetroxide with thionyl chloride :[4]
OsO4 2 4 2 2
Osmium tetraoxide dissolves in hydrochloric acid to give the hexachloroosmate anion:
OsO4 2 6 2 2 O
References
[ edit ]
^ Cotton, S. A. (1997). Chemistry of Precious Metals . London: Chapman and Hall. ISBN 0-7514-0413-6
^ Wells A.F. (1984). Structural Inorganic Chemistry (5th ed.). Oxford Science Publications. ISBN 0-19-855370-6
^ Paul Machmer (1967). "On the polymorphism of osmium tetrachloride". Chem. Commun. 12 ): 610a. doi :10.1039/C1967000610A .
t
e
Os(0)
Os(0,I)
Os(I )
Os(I,II)
Os(II )
Os(III)
Os(IV )
Os(V )
Os(VI )
Os(VII)
Os(VIII)
t
e
Salts and covalent derivatives of the
chloride ion
R e t r i e v e d f r o m " https://en.wikipedia.org/w/index.php?title=Osmium(IV )_chloride&oldid=1144335996 " C a t e g o r i e s : ● O s m i u m c o m p o u n d s ● C h l o r i d e s ● P l a t i n u m g r o u p h a l i d e s H i d d e n c a t e g o r i e s : ● C h e m i c a l p a g e s w i t h o u t C h e m S p i d e r I D ● A r t i c l e s w i t h o u t E B I s o u r c e ● A r t i c l e s w i t h o u t K E G G s o u r c e ● E C H A I n f o C a r d I D f r o m W i k i d a t a ● A r t i c l e s w i t h c h a n g e d F D A i d e n t i f i e r ● A r t i c l e s c o n t a i n i n g u n v e r i f i e d c h e m i c a l i n f o b o x e s ● A r t i c l e s w i t h s h o r t d e s c r i p t i o n ● S h o r t d e s c r i p t i o n m a t c h e s W i k i d a t a
● T h i s p a g e w a s l a s t e d i t e d o n 1 3 M a r c h 2 0 2 3 , a t 0 4 : 5 5 ( U T C ) . ● T e x t i s a v a i l a b l e u n d e r t h e C r e a t i v e C o m m o n s A t t r i b u t i o n - S h a r e A l i k e L i c e n s e 4 . 0 ;
a d d i t i o n a l t e r m s m a y a p p l y . B y u s i n g t h i s s i t e , y o u a g r e e t o t h e T e r m s o f U s e a n d P r i v a c y P o l i c y . W i k i p e d i a ® i s a r e g i s t e r e d t r a d e m a r k o f t h e W i k i m e d i a F o u n d a t i o n , I n c . , a n o n - p r o f i t o r g a n i z a t i o n . ● P r i v a c y p o l i c y ● A b o u t W i k i p e d i a ● D i s c l a i m e r s ● C o n t a c t W i k i p e d i a ● C o d e o f C o n d u c t ● D e v e l o p e r s ● S t a t i s t i c s ● C o o k i e s t a t e m e n t ● M o b i l e v i e w