ドーパミン
表示
(ドパミンから転送)
この記事は英語版の対応するページを翻訳することにより充実させることができます。(2023年10月) 翻訳前に重要な指示を読むには右にある[表示]をクリックしてください。
●英語版記事を日本語へ機械翻訳したバージョン︵Google翻訳︶。
●万が一翻訳の手がかりとして機械翻訳を用いた場合、翻訳者は必ず翻訳元原文を参照して機械翻訳の誤りを訂正し、正確な翻訳にしなければなりません。これが成されていない場合、記事は削除の方針G-3に基づき、削除される可能性があります。
●信頼性が低いまたは低品質な文章を翻訳しないでください。もし可能ならば、文章を他言語版記事に示された文献で正しいかどうかを確認してください。
●履歴継承を行うため、要約欄に翻訳元となった記事のページ名・版について記述する必要があります。記述方法については、Wikipedia:翻訳のガイドライン#要約欄への記入を参照ください。
●翻訳後、 {{翻訳告知|en|Dopamine|…}}をノートに追加することもできます。
●Wikipedia:翻訳のガイドラインに、より詳細な翻訳の手順・指針についての説明があります。
|
| ドーパミン | |
|---|---|

| |
| |
4-(2-アミノエチル)ベンゼン-1,2-ジオール | |
別称 ドパミン, DA 2-(3,4-ジヒドロキシフェニル)エチルアミン 3,4-ジヒドロキシフェネチルアミン 3-ヒドロキシチラミン Intropin Revivan オキシチラミン | |
| 識別情報 | |
| CAS登録番号 | 51-61-6 |
| KEGG | D07870 |
| |
| 特性 | |
| 化学式 | C8H11NO2 |
| モル質量 | 153.178 g/mol |
| 融点 |
128 ℃ (401 K) |
| 特記なき場合、データは常温 (25 °C)・常圧 (100 kPa) におけるものである。 | |
ドーパミン︵英: dopamine︶は、中枢神経系に存在する神経伝達物質で、アドレナリン、ノルアドレナリンの前駆体でもある。運動調節、ホルモン調節、快の感情、意欲、学習などに関わる。セロトニン、ノルアドレナリン、アドレナリン、ヒスタミン、ドーパミンを総称してモノアミン神経伝達物質と呼ぶ。またドーパミンは、ノルアドレナリン、アドレナリンと共にカテコール基をもつためカテコールアミンとも総称される。医学・医療分野では日本語表記をドパミンとしている[1]。合成された注射製剤が循環器科[2]、救急[3]、集中治療医学[2]、麻酔科学[4]等の領域で頻用されている。

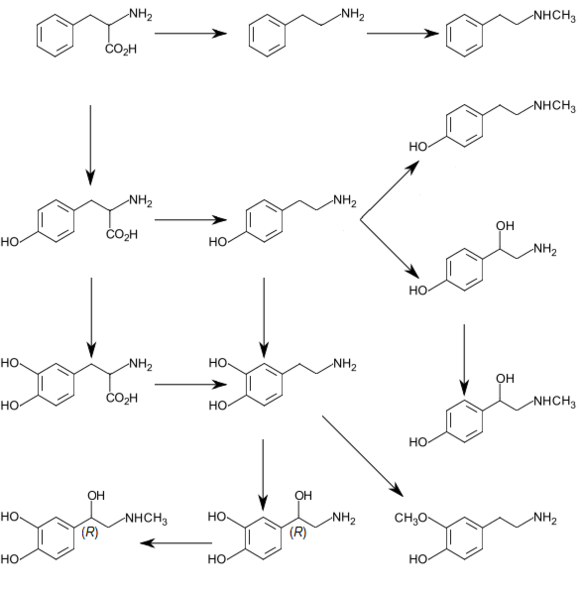
ドーパミンの生合成経路
ドーパミンの前駆体はL-ドーパである。L-ドーパはフェニルアラニンやチロシンの水酸化によって作られる。
●チロシン→L-ドーパ︵L-ジヒドロキシフェニルアラニン︶
●チロシン水酸化酵素 (tyrosine hydroxylase, TH) EC1.14.16.2
●L-ドーパ→ドーパミン
●ドーパ脱炭酸酵素(DOPA decarboxylase︶EC 4.1.1.28
さらに一部のニューロンにおいては、ドーパミンから、ドーパミン-β-モノオキシゲナーゼ (dopamine beta hydroxylase, DBH; あるいは dopamine beta-monooxygenase) EC1.14.17.1によってノルアドレナリンが合成される。
概要[編集]
統合失調症の陽性症状︵幻覚・妄想など︶は基底核や中脳辺縁系ニューロンのドーパミン過剰によって生じるという仮説がある。この仮説に基づき薬物療法で一定の成果を収めてきているが、一方で陰性症状には効果が無く、根本的病因としては仮説の域を出ていない。覚醒剤はドーパミン作動性に作用するため、中毒症状は統合失調症に類似する。強迫性障害、トゥレット障害、注意欠陥多動性障害 (ADHD) においてもドーパミン機能の異常が示唆されている。 一方、パーキンソン病では黒質線条体のドーパミン神経が減少し筋固縮、振戦、無動などの運動症状が起こる。また抗精神病薬などドーパミン遮断薬の副作用としてパーキンソン症候群が起こることがある。 中脳皮質系ドーパミン神経は、とくに前頭葉に分布するものが報酬系などに関与し、意欲、動機、学習などに重要な役割を担っていると言われている。新しい知識が長期記憶として貯蔵される際、ドーパミンなどの脳内化学物質が必要になる[5]。陰性症状の強い統合失調症患者や、一部のうつ病では前頭葉を中心としてドーパミンD1の機能が低下しているという仮説がある。 下垂体漏斗系においてドーパミンはプロラクチンなどの分泌抑制因子として働く。そのためドーパミン作動薬は高プロラクチン血症の治療薬として使用され、逆にドーパミン遮断薬︵抗精神病薬など︶は副作用として高プロラクチン血症を誘発する。 ドーパミン部分作動薬のアリピプラゾール︵エビリファイ︶は低プロラクチン血症を誘発することが分かっており[6]、高プロラクチン血症の治療効果もある。その副作用として異常性欲や性的倒錯があり[7]、アメリカ食品医薬品局︵FDA︶は添付文書で黒枠の警告をしている[8]。生合成過程[編集]

放出・再取り込み・分解[編集]
ニューロンでは、ドーパミンは合成された後、小胞の中へ充填され︵中枢神経系では小胞性モノアミン輸送体2 vesicular monoamine transporter 2 (VMAT2, SLC18A2) の働きによる︶、活動電位の発生に伴って、放出される。
放出後のドーパミンは、ドーパミン輸送体 (dopamine transporter, DAT, SLC6A3) によって、ドーパミン作動性の軸索に再取り込みされる。その後、カテコール-O-メチル基転移酵素 (catechol-O-methyl transferase, COMT) EC2.1.1.6およびモノアミン酸化酵素 (monoamine oxidase, MAO) EC1.4.3.4によって、分解される。酵素による分解を免れたドーパミンは、再び小胞へと充填されて再利用されると考えられている。
薬剤[編集]
ドーパミンが関係する薬剤には以下のようなものがある。抗精神病薬は、主にドーパミンD2受容体を遮断することで効果を発現する。抗パーキンソン病薬のほとんどは、ドーパミンの前駆体であったりドーパミン受容体を刺激したりすることでドーパミン作動性に働くことで効果を発現する。
末梢において作用するもの
ドーパミン︵イノバン、カタボン︶‥急性循環不全治療薬
ドーパミン作動薬︵アゴニスト︶
L-ドーパ︵ドパストン︶、L-ドパ・カルビドパ配合剤︵ネオドパストン︶、カベルゴリン︵カバサール︶、ブロモクリプチン︵パーロデル︶、ケタミン︵ケタラール︶、メマンチン︵メマリー︶、アマンタジン︵シンメトレル︶、メチルフェニデート︵リタリン、コンサータ︶、アンフェタミン︵Adderall︶、メタンフェタミン、コカイン、ジゾシルピン︵MK-801︶など。
ドーパミン部分作動薬︵パーシャルアゴニスト︶
アリピプラゾール︵エビリファイ︶、フェンサイクリジン︵PCP︶など。
ドーパミン拮抗剤︵アンタゴニスト︶
抗精神病薬︵クロルプロマジン、ハロペリドール、オランザピンなど︶
ドーパミン放出阻害剤
ミノサイクリン︵ミノマイシン︶[12]
その他
スルピリド
脚注[編集]
- ^ 日本神経学会用語委員会編『神経学用語集 改訂第3版』文光堂、2008年、p.42
- ^ a b Suzuki, Reina; Uchino, Shigehiko; Sasabuchi, Yusuke; Kawarai Lefor, Alan; Sanui, Masamitsu (2022-04-02). “Dopamine use and its consequences in the intensive care unit: a cohort study utilizing the Japanese Intensive care PAtient Database”. Critical Care 26 (1): 90. doi:10.1186/s13054-022-03960-y. ISSN 1364-8535. PMC PMC8977005. PMID 35366934.
- ^ RaaeNielsen, Jen; Fales, William (2017-01-01). “Use of Dopamine in a Statewide Emergency Medical Services System”. Research Day.
- ^ “麻酔薬および麻酔関連薬使用ガイドライン 第3版 Ⅶ 循環作動薬”. 公益社団法人日本麻酔科学会. p. 231. 2023年9月23日閲覧。
- ^ HARVARD MEDICAL SCHOOL (2016年10月). “Need to remember something? Exercise four hours later”. 2017年9月2日閲覧。
- ^ Sogawa R, Shimomura Y, Minami C, Maruo J, Kunitake Y, Mizoguchi Y, Kawashima T, Monji A, Hara H. (2016-8). “Aripiprazole-Associated Hypoprolactinemia in the Clinical Setting.”. en:Journal of Clinical Psychopharmacology. 36 (4): 385-7. doi:10.1097/JCP.0000000000000527. PMID 27281387.
- ^ Vrignaud L, Aouille J, Mallaret M, Durrieu G, Jonville-Béra AP. (2014-11-1). “Hypersexuality associated with aripiprazole: a new case and review of the literature.”. Therapie. 69 (6): 525-527. doi:10.2515/therapie/2014064. PMID 25293487.
- ^ FDA Drug Safety Communication: FDA warns about new impulse-control problems associated with mental health drug aripiprazole (Abilify, Abilify Maintena, Aristada) (05-03-2016 FDA)
- ^ Broadley KJ (March 2010). “The vascular effects of trace amines and amphetamines”. Pharmacol. Ther. 125 (3): 363–375. doi:10.1016/j.pharmthera.2009.11.005. PMID 19948186.
- ^ “A renaissance in trace amines inspired by a novel GPCR family”. Trends Pharmacol. Sci. 26 (5): 274–281. (May 2005). doi:10.1016/j.tips.2005.03.007. PMID 15860375.
- ^ “The endogenous substrates of brain CYP2D”. Eur. J. Pharmacol. 724: 211–218. (February 2014). doi:10.1016/j.ejphar.2013.12.025. PMID 24374199. "The highest level of brain CYP2D activity was found in the substantia nigra ... The in vitro and in vivo studies have shown the contribution of the alternative CYP2D-mediated dopamine synthesis to the concentration of this neurotransmitter although the classic biosynthetic route to dopamine from tyrosine is active. ... Tyramine levels are especially high in the basal ganglia and limbic system, which are thought to be related to individual behavior and emotion (Yu et al., 2003c). ... Rat CYP2D isoforms (2D2/2D4/2D18) are less efficient than human CYP2D6 for the generation of dopamine from p-tyramine. The Km values of the CYP2D isoforms are as follows: CYP2D6 (87–121 μm) ≈ CYP2D2 ≈ CYP2D18 > CYP2D4 (256 μm) for m-tyramine and CYP2D4 (433 μm) > CYP2D2 ≈ CYP2D6 > CYP2D18 (688 μm) for p-tyramine"
- ^ Lin Zhang, Yukihiko Shirayama, Masaomi Iyo, Kenji Hashimoto. (2007-9). “Minocycline attenuates hyperlocomotion and prepulse inhibition deficits in mice after administration of the NMDA receptor antagonist dizocilpine.”. en:Neuropsychopharmacology (journal). 32 (9). doi:10.1038/sj.npp.1301313. PMID 17228338.
関連項目[編集]
関連人物[編集]
外部リンク[編集]
- Dopamine Modulation (英語) - スカラーペディア百科事典「ドーパミンによる神経修飾」の項目。
- ドーパミン - 脳科学辞典
- ドーパミン仮説 - 脳科学辞典