

 | |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider |
|
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.035.274 |
| Chemical and physical data | |
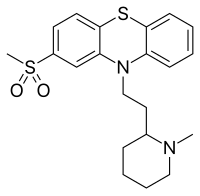
| Formula | C21H26N2O2S2 |
| Molar mass | 402.57 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Sulforidazine (Imagotan, Psychoson, Inofal) a typical antipsychotic and a metaboliteofthioridazine; it and mesoridazine are more potent than the parent compound, whose pharmacological effects are believed by some to be largely due to its metabolism into sulforidazine and mesoridazine.[1]

2-bromo-2'-amino-4'-methylsulphonyl-diphenyl Sulphide, CID:43448246 (1) 2-bromo-2'-acetamino-4'-methylsulphonyl diphenylsulphide (2) 2-(2-Chloroethyl)-1-Methylpiperidine [50846-01-0] (3)
| |||||||||||||||||||||||||
|
| |||||||
|---|---|---|---|---|---|---|---|
| D1-like |
| ||||||
| D2-like |
| ||||||
| |||||||
|
| |||||
|---|---|---|---|---|---|
| H1 |
| ||||
| H2 |
| ||||
| H3 |
| ||||
| H4 |
| ||||
| |||||
|
| |
|---|---|
| Classes |
|
| Antidepressants (Tricyclic antidepressants (TCAs)) |
|
| Antihistamines |
|
| Antipsychotics |
|
| Anticonvulsants |
|
| Anticholinergics |
|
| Others |
|
This drug article relating to the nervous system is a stub. You can help Wikipedia by expanding it. |