

 | |
| Clinical data | |
|---|---|
| Trade names | Glufast |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | By mouth (tablets) |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank |
|
| ChemSpider |
|
| UNII | |
| KEGG |
|
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
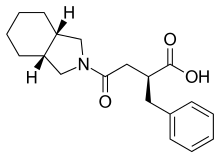
| Formula | C19H25NO3 |
| Molar mass | 315.413 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Mitiglinide (INN,[1] trade name Glufast) is a drug for the treatment of type 2 diabetes.[2]
Mitiglinide belongs to the meglitinide (glinide) class of blood glucose-lowering drugs and is currently co-marketed in Japan by Kissei and Takeda. The North America rights to mitiglinide are held by Elixir Pharmaceuticals. Mitiglinide has not yet gained FDA approval.
Mitiglinide is thought to stimulate insulin secretion by closing the ATP-sensitive potassium KATP channels in pancreatic β cells.
Mitiglinide is delivered in tablet form.
|
| |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Calcium |
| ||||||||||||||||||||||||
| Potassium |
| ||||||||||||||||||||||||
| Sodium |
| ||||||||||||||||||||||||
| Chloride |
| ||||||||||||||||||||||||
| Others |
| ||||||||||||||||||||||||
See also: Receptor/signaling modulators • Transient receptor potential channel modulators | |||||||||||||||||||||||||