

 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | spar FLOX a sin |
| Trade names | Spacin, Zagam, others |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| MedlinePlus | a600002 |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 92% |
| Protein binding | 45% |
| Metabolism | Hepatic glucuronidation Cytochrome P450 system not involved |
| Elimination half-life | 16 to 30 hours |
| Excretion | Fecal (50%) and renal (50%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank |
|
| ChemSpider |
|
| UNII | |
| KEGG |
|
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.157.238 |
| Chemical and physical data | |
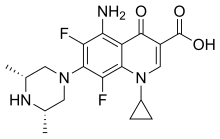
| Formula | C19H22F2N4O3 |
| Molar mass | 392.407 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 266 to 269 °C (511 to 516 °F) (dec.) |
| |
| |
| (verify) | |
Sparfloxacin is a fluoroquinolone antibiotic used in the treatment of bacterial infections. It has a controversial safety profile.[1]
It was patented in 1985 and approved for medical use in 1993.[2] Zagam is no longer available in the United States.
The compound is indicated for treating community-acquired lower respiratory tract infections (acute sinusitis, exacerbations of chronic bronchitis caused by susceptible bacteria, community-acquired pneumonia).[3][4][5][6]
Sparfloxacin is about 37-45% bound to proteins in the blood.[11][12]
Shimada et al. ( 1993) has summarized many of the studies published in Japanese regarding the tissue distribution of sparfloxacin. (high concentrations are achieved in sputum, pleural fluid, skin, lung, prostate, gynecological tissues, breast milk and otolaryngological tissues. *Salivary concentrations are 66 to 70% of plasma levels, while CSF penetration appears to be somewhat limited with CSF:plasma concentration ratios of only 0.25 to 0.35.
In rabbits, sparfloxacin achieves very good penetration into the ocular vitreous (54%), cornea (76%) and lens (36%).[17]
Sparfloxacin, like other quinolones and fluoroquinolones, are bactericidal drugs, actively killing bacteria. Quinolones inhibit the bacterial DNA gyrase or the topoisomerase IV enzyme, thereby inhibiting DNA replication and transcription. Quinolones can enter cells easily and therefore are often used to treat intracellular pathogens such as Legionella pneumophila and Mycoplasma pneumoniae. For many gram-negative bacteria DNA gyrase is the target, whereas topoisomerase IV is the target for many gram-positive bacteria. Eukaryotic cells do not contain DNA gyraseortopoisomerase IV.
|
| |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antifolates (inhibit bacterial purine metabolism, thereby inhibiting DNA and RNA synthesis) |
| ||||||||||||||||
| Quinolones (inhibit bacterial topoisomerase and/or DNA gyrase, thereby inhibiting DNA replication) |
| ||||||||||||||||
| Anaerobic DNA inhibitors |
| ||||||||||||||||
| RNA synthesis |
| ||||||||||||||||
| |||||||||||||||||