

 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider |
|
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
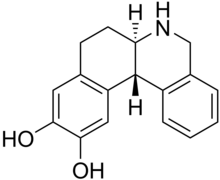
| Formula | C17H17NO2 |
| Molar mass | 267.328 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Dihydrexidine (DAR-0100) is a moderately selective full agonist at the dopamine D1 and D5 receptors.[1] It has approximately 10-fold selectivity for D1 and D5 over the D2 receptor.[2] Although dihydrexidine has some affinity for the D2 receptor, it has functionally selective (highly biased) D2 signaling,[3] thereby explaining why it lacks D2 agonist behavioral qualities.[4]
Dihydrexidine has shown impressive antiparkinson effects in the MPTP-primate model,[5] and has been investigated for the treatmentofParkinson's disease.[6] In an early clinical trial the drug was given intravenously and led to profound hypotensionsodevelopment was halted.[7] The drug was resurrected when it was shown that smaller subcutaneous doses were safe.[8] This led to a pilot study in schizophrenia[9] and current clinical trials to assess its efficacy in improving the cognitive and working memory deficitsinschizophrenia and schizotypal disorder.
There have been several reviews of relevance to the compound.[10][11][12]
|
| |||||
|---|---|---|---|---|---|
| Sympatholytics (antagonize α-adrenergic vasoconstriction) |
| ||||
| Other antagonists |
| ||||
| |||||
|
| |||||||
|---|---|---|---|---|---|---|---|
| D1-like |
| ||||||
| D2-like |
| ||||||
| |||||||
This antihypertensive-related article is a stub. You can help Wikipedia by expanding it. |