

| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2-Oxopropanoic acid[1] | |||
| Systematic IUPAC name
2-Oxopropionic acid | |||
| Other names
Pyruvic acid[1] | |||
| Identifiers | |||
| |||
3D model (JSmol) |
|||
| Abbreviations | Pyr | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider |
| ||
| DrugBank |
| ||
| ECHA InfoCard | 100.004.387 | ||
| KEGG |
| ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C3H4O3 | |||
| Molar mass | 88.06 g/mol | ||
| Density | 1.250 g/cm3 | ||
| Melting point | 11.8 °C (53.2 °F; 284.9 K) | ||
| Boiling point | 165 °C (329 °F; 438 K) | ||
| Acidity (pKa) | 2.50[2] | ||
| Related compounds | |||
Other anions |
Pyruvate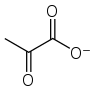 
| ||
Related keto-acids, carboxylic acids |
| ||
Related compounds |
| ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
Pyruvic acid (IUPAC name: 2-oxopropanoic acid, also called acetoic acid) (CH3COCOOH) is the simplest of the alpha-keto acids, with a carboxylic acid and a ketone functional group. Pyruvate, the conjugate base, CH3COCOO−, is an intermediate in several metabolic pathways throughout the cell.
Pyruvic acid can be made from glucose through glycolysis, converted back to carbohydrates (such as glucose) via gluconeogenesis, or converted to fatty acids through a reaction with acetyl-CoA.[3] It can also be used to construct the amino acid alanine and can be converted into ethanolorlactic acid via fermentation.
Pyruvic acid supplies energy to cells through the citric acid cycle (also known as the Krebs cycle) when oxygen is present (aerobic respiration), and alternatively ferments to produce lactate when oxygen is lacking.[4]
In 1834, Théophile-Jules Pelouze distilled tartaric acid and isolated glutaric acid and another unknown organic acid. Jöns Jacob Berzelius characterized this other acid the following year and named pyruvic acid because it was distilled using heat.[5][6] The correct molecular structure was deduced by the 1870s.[7]
Pyruvic acid is a colorless liquid with a smell similar to that of acetic acid and is miscible with water.[8] In the laboratory, pyruvic acid may be prepared by heating a mixture of tartaric acid and potassium hydrogen sulfate,[9] by the oxidationofpropylene glycol by a strong oxidizer (e.g., potassium permanganateorbleach), or by the hydrolysis of acetyl cyanide, formed by reaction of acetyl chloride with potassium cyanide:[citation needed]
This article needs additional citations for verification. Please help improve this articlebyadding citations to reliable sources. Unsourced material may be challenged and removed.
Find sources: "Pyruvic acid" – news · newspapers · books · scholar · JSTOR (December 2023) (Learn how and when to remove this message) |
Pyruvate is an important chemical compoundinbiochemistry. It is the output of the metabolism of glucose known as glycolysis.[10] One molecule of glucose breaks down into two molecules of pyruvate,[10] which are then used to provide further energy, in one of two ways. Pyruvate is converted into acetyl-coenzyme A, which is the main input for a series of reactions known as the Krebs cycle (also known as the citric acid cycle or tricarboxylic acid cycle). Pyruvate is also converted to oxaloacetate by an anaplerotic reaction, which replenishes Krebs cycle intermediates; also, the oxaloacetate is used for gluconeogenesis.[citation needed]
These reactions are named after Hans Adolf Krebs, the biochemist awarded the 1953 Nobel Prize for physiology, jointly with Fritz Lipmann, for research into metabolic processes. The cycle is also known as the citric acid cycle or tricarboxylic acid cycle, because citric acid is one of the intermediate compounds formed during the reactions.[citation needed]
If insufficient oxygen is available, the acid is broken down anaerobically, creating lactate in animals and ethanol in plants and microorganisms (and carp[11]). Pyruvate from glycolysis is converted by fermentationtolactate using the enzyme lactate dehydrogenase and the coenzyme NADH in lactate fermentation, or to acetaldehyde (with the enzyme pyruvate decarboxylase) and then to ethanolinalcoholic fermentation.[citation needed]
Pyruvate is a key intersection in the network of metabolic pathways. Pyruvate can be converted into carbohydrates via gluconeogenesis, to fatty acids or energy through acetyl-CoA, to the amino acid alanine, and to ethanol. Therefore, it unites several key metabolic processes.[citation needed]

In the last step of glycolysis, phosphoenolpyruvate (PEP) is converted to pyruvate by pyruvate kinase. This reaction is strongly exergonic and irreversible; in gluconeogenesis, it takes two enzymes, pyruvate carboxylase and PEP carboxykinase, to catalyze the reverse transformation of pyruvate to PEP.[citation needed]
| phosphoenolpyruvate | pyruvate kinase | pyruvic acid | |

|

| ||
| ADP | ATP | ||
| |||
| ADP | ATP | ||
| pyruvate carboxylase and PEP carboxykinase | |||
Compound C00074atKEGG Pathway Database. Enzyme 2.7.1.40atKEGG Pathway Database. Compound C00022atKEGG Pathway Database.
Click on genes, proteins and metabolites below to link to respective articles.[§ 1]
Pyruvate decarboxylation by the pyruvate dehydrogenase complex produces acetyl-CoA.
| pyruvate | pyruvate dehydrogenase complex | acetyl-CoA | |

|

| ||
| CoA + NAD+ | CO2 + NADH +H+ | ||
| |||
Carboxylation by pyruvate carboxylase produces oxaloacetate.
| pyruvate | pyruvate carboxylase | oxaloacetate | |

|
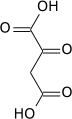
| ||
| ATP +CO2 | ADP +Pi | ||

| |||
Transamination by alanine transaminase produces alanine.
| pyruvate | alanine transaminase | alanine | |

|

| ||
| glutamate | α-ketoglutarate | ||
| |||
| glutamate | α-ketoglutarate | ||
Reduction by lactate dehydrogenase produces lactate.
| pyruvate | lactate dehydrogenase | lactate | |

|

| ||
| NADH | NAD+ | ||
| |||
| NADH | NAD+ | ||
Pyruvic acid is an abundant carboxylic acid in secondary organic aerosols.[12]
Pyruvate is sold as a weight-loss supplement, though credible science has yet to back this claim. A systematic review of six trials found a statistically significant difference in body weight with pyruvate compared to placebo. However, all of the trials had methodological weaknesses and the magnitude of the effect was small. The review also identified adverse events associated with pyruvate such as diarrhea, bloating, gas, and increase in low-density lipoprotein (LDL) cholesterol. The authors concluded that there was insufficient evidence to support the use of pyruvate for weight loss.[13]
There is also in vitro as well as in vivo evidence in hearts that pyruvate improves metabolism by NADH production stimulation and increases cardiac function.[14][15]
{{cite book}}: CS1 maint: DOI inactive as of April 2024 (link)
|
| |
|---|---|
|
ATP ADP
ATP ADP
+ +
NAD++ Pi NADH + H+ NAD++ Pi NADH + H+ ADP ATP ADP ATP
H2O
H2O ADP ATP
2 × Pyruvate |
|
| |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K→acetyl-CoA |
| ||||||||||||||||||||||||||||||||
| G |
| ||||||||||||||||||||||||||||||||
| Other |
| ||||||||||||||||||||||||||||||||