ファビピラビル
(アビガンから転送)
 | |
| IUPAC命名法による物質名 | |
|---|---|
| |
| 臨床データ | |
| 販売名 | Avigan(アビガンAbigan) |
| 法的規制 |
|
| 識別 | |
| CAS番号 | 259793-96-9 |
| ATCコード | J05AX27 (WHO) |
| PubChem | CID: 492405 |
| DrugBank | DB12466 |
| ChemSpider | 431002 |
| UNII | EW5GL2X7E0 |
| KEGG | D09537 |
| ChEBI | CHEBI:134722 |
| ChEMBL | CHEMBL221722 |
| 別名 | T-705, favipira, favilavir |
| 化学的データ | |
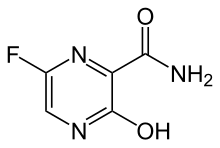
| 化学式 | C5H4FN3O2 |
| 分子量 | 157.10 g·mol−1 |
| |
| |
ファビピラビル︵英: Favipiravir、中: 法匹拉韦、法匹拉韋︶は、富山大学医学部教授の白木公康と富士フイルムホールディングス傘下の富山化学工業︵現‥富士フイルム富山化学︶が共同研究で開発した核酸アナログでRNA依存性RNAポリメラーゼ阻害剤[1]である。開発コードのT-705、あるいは商品名であるアビガン︵Avigan、登録商標第4500382号ほか︶[注 1]の名前でも呼ばれる。
中華人民共和国では、浙江海正薬業股份有限公司がライセンスを取得して生産していた。ただし、2019年に中国における富士フイルムのファビピラビルの物質特許は失効しており、それ以降はライセンスによらずに、後発医薬品︵商品名﹁法维拉韦﹂︶として製造している[3][4]。
作用機序[編集]
ファビピラビルは、富山化学工業の江川裕之らが合成し、古田要介らが抗インフルエンザ活性を見出した。富山大学医学部の白木公康らがインフルエンザ感染マウスでの有効性を確認し、タミフルより強い治療効果を有していること、薬剤耐性を生じないことを見出した。
ファビピラビルはプロドラッグであり、投与後に細胞内のin vivo︵生体内︶環境で、三リン酸化されて、ファビピラビル・リボフラノシル-5'-三リン酸 (favipiravir-RTP) となり、これがRNAウイルスのRNA依存性RNAポリメラーゼ︵RdRP、RNA複製酵素︶にプリンヌクレオシド︵アデノシンおよびグアノシン︶と競合して取り込まれ、取り込まれた部位以降のRNA鎖の伸長を阻害するchain terminator︵伸長阻止薬︶として作用する (Furuta, Shiraki et al., 2013 [5])[6]。
in vitro︵試験管内︶環境では、ファビピラビルは十分な三リン酸化を受けることができないため、50%効果濃度︵EC50値︶などを基準とした効果評価においては、他の薬物より効果が劣ると判定される場合がある︵エボラ出血熱ウイルス、2019新型コロナウイルス︶。
抗インフルエンザウイルス薬としては、細胞内におけるウイルスRNAの複製を妨げることで増殖を防ぐ仕組みで、タミフルなどの既存薬とは作用機序が異なる。そのためインフルエンザウイルスの種類を問わず抗ウイルス作用が期待できる[7]︵たとえばタミフルはB型インフルエンザウイルスに対しては効果が劣り、C型インフルエンザウイルスには効果がない︶。
また、インフルエンザウイルスのみならず、エボラ出血熱ウイルス︵後述︶やノロウイルス、SFTSウイルス︵後述︶などへの適用性に関する試験・研究も行われており、ケンブリッジ大学教授のイアン・グッドフェローらの研究チームは2014年10月21日、マウスを使った実験でノロウイルスの減少・消失を確認したとの発表を行った[8][9][10]。さらにウエストナイル熱ウイルス、黄熱ウイルスなどのRNAウイルスにも効果があると考えられており、同研究チームは﹁治療だけでなく、感染予防にも効果的である可能性がある﹂とコメントしている[11]。
承認[編集]
日本以外で承認されている国・地域は無かった[12]が、2020年には新型コロナウイルス感染症 (COVID-19) の治療に効果があるとして各国で研究・治験が開始された。2020年2月22日、厚生労働大臣加藤勝信は、新型コロナウイルス感染症︵後述︶の治療の一環として、投与する考えを示した[13]。日本[編集]
2014年︵平成26年︶3月に、富山化学工業が日本での製造販売承認を取得した[1]。ただしすぐに製造・販売が開始される訳ではなく、新型インフルエンザが流行し、他の薬剤が効かないと日本国政府が判断した場合に、厚生労働大臣の要請を受けて製造を開始するという特殊な承認となっている[7]。 富山化学工業は当初、アビガンがタミフルに代わる新しいインフルエンザ薬として普及し、会社の収益源となることを期待していたが、動物実験で胎児に対する催奇形性の可能性が指摘されたため、厚生労働省による製造販売承認は大幅に遅れたうえに、緊急の場合のみ製造可能という条件がついてしまい、経営に貢献するという期待は外れる結果になった[14]。 臨床試験の結果からインフルエンザに対する有効性が示されなかったことが判明、加えて催奇形性の危険があるにもかかわらず承認されたのは、既存の抗インフルエンザウイルス薬は、ウイルスを細胞内に閉じ込めることで感染細胞を増やさない働きをするのに対し、アビガンはウイルスの遺伝子複製そのものを阻害するため、作用機序が全く異なり、既存薬に耐性を有するウイルスが蔓延した場合の備えになると期待されたためである[14]。ウイルスの遺伝子複製そのものを阻害する抗インフルエンザウイルス薬としては後にエンドヌクレアーゼ阻害薬のゾフルーザが上市されたが、こちらは高額な上に耐性ウイルスが生じやすい懸念があるため、採用を見送る医療機関も出ている[15]。新型インフルエンザの流行に備え備蓄[編集]
2017年3月9日、厚生労働省は、新型インフルエンザの流行に備えアビガン錠を備蓄することを決め、2017年度に3万人分を発注する随意契約を3月30日に結ぶ方針であることを発表した[16]。同月30日、日本国政府の新型インフルエンザ等対策有識者会議は、約200万人分を上限目標に備蓄することとし、厚生労働省は同日、約4万7000人分のアビガンを購入する契約を富山化学と結んだ[17]。 日本国政府が備蓄しているアビガン錠の放出は、﹁国の指示に基づき指定された医療機関へ放出﹂﹁新型インフルエンザ発生後速やかに、安全性及び有効性の知見・情報を集積する体制︵臨床試験等︶を整備﹂とされている[18]。特定および第一種感染症指定医療機関または当該都道府県において、都道府県知事が指定する医療機関が供給依頼し、厚生労働省が富士フイルム富山化学に出庫指示を出す[19]。特許について[編集]
2020年3月18日付けの日経バイオテクによれば、2019年に中華人民共和国における富士フイルムのファビピラビルの物質特許は失効している。ただし、製造特許は存続している[4]。日本における物質特許は、富士フイルムが5年間の延長手続きを行っており、2024年まで有効である[20]。 中国・浙江海正薬業股份有限公司︵Zhejiang Hisun Pharmaceutical︶は、2016年6月、富士フイルムとファビピラビルの特許ライセンス契約を締結し、中国における製造、販売のライセンスを取得している。富士フイルムは、一時金やロイヤルティを受け取る契約であった。2019年の富士フイルムの物質特許の失効に伴って、このライセンス契約も終了し、その後は、浙江海正薬業は、富士フイルムの製造特許に触れない形で、後発医薬品としてファビピラビルを製造しており、これらについては、富士フイルムはロイヤルティを受け取ることはできない。中国で行われた2019新型コロナウイルスの国家主導臨床試験に提供された、浙江海正薬業が製造したファビピラビルも、この後発医薬品に該当する[4]。 なお、富士フイルムの広報担当者は、﹁現在もZhejiang Hisun Pharmaceutical社とは協力関係にあるものの、製造特許は存続しているため、︵それを回避する方法で製造されている同社の製品は︶不純物の組成などが富士フイルムが製造するファビピラビルと若干異なる可能性は否定はできない﹂とコメントしている[4]。薬価[編集]
日本[編集]
2020年4月の段階では、流通していないため、薬価も調剤報酬も設定されていない[21]。 ただし、厚生労働省は備蓄用のアビガン錠を数回に亘って随意契約で購入しており、落札価格が開示されているので、そこから購入単価を推定することは可能である。例えば平成29年度︵2017年度︶の随意契約﹁抗インフルエンザウイルス薬︵アビガン錠200mg︶3万人分の購入﹂では、平成29年6月1日付の官報に、落札価格が158,950,000円と公示されており [22]、これから計算すれば、1人当たり約5,298.3円、抗インフルエンザ用途の場合の1人当たりアビガン投与量を﹁200mg錠×40錠﹂とすれば、1錠約132.5円となる。 なお、アビガン錠のように一般に流通せず、日本国政府の備蓄のみとなっている処方箋医薬品は前例がないため、新型コロナウイルス感染症の治療薬として承認されて、薬局に流通可能となった場合の薬価が、この購入価格と同程度になる保証はない。インド[編集]
2020年6月20日の報道によると、Glenmarkが開発したジェネリックであるファビピラビル︵販売名‥FabiFlu®︶200 mg錠の最大小売価格︵Maximum Retail Price‥MRP︶は、34錠のストリップ包装当たり3,500ルピー(約5,000円)としている[23]。 2020年7月24日、Jenburkt Pharmaceuticals Ltdは、ジェネリックであるファビピラビル︵販売名‥Favivent︶200 mg錠を、1錠あたり39ルピーで発売すると発表し[24]、Cipla Ltdは、ジェネリックであるファビピラビル︵販売名‥Ciplenza︶200 mg錠を、8月第1週に、1錠あたり68ルピーで発売すると発表した[25]。 2020年8月4日、サンファーマは、ジェネリックであるファビピラビル︵販売名‥FluGuard︶200 mg錠を、1錠あたり35ルピーで発売すると発表した[26]。研究事例[編集]
H7N9鳥インフルエンザへの適用[編集]
2017年2月21日、台湾の衛生福利部疾病管制署︵台湾CDC︶は、輸入していたファビピラビルを、薬剤耐性のH7N9亜型インフルエンザ感染患者のために放出したと発表した[27][28]。同月27日、この患者は多臓器不全で死亡した[29]。マダニ感染症への適用[編集]
2016年2月22日、厚生労働省研究班のチームがマダニが媒介するウイルス感染症﹁重症熱性血小板減少症候群 (SFTS)﹂に、ファビピラビルが有効であることをマウスの実験で確かめたと、米微生物学会の専門誌に発表した[30]。感染直後に投与すれば高い確率で救命できることが示唆された[30]。2016年6月より愛媛大学、長崎大学、国立国際医療研究センター、国立感染症研究所など日本国内30ヵ所の医療機関が臨床研究を開始した[31][32][33]。 2017年11月9日、愛媛大学らの研究グループが、﹁一定の治療効果が認められた﹂と臨床研究の結果を発表した[34]。同グループは今後治療法の確立を目指すとしている[34]。 2018年3月12日、富山化学がマダニによるSFTSに対する治験の最終段階である第III相試験︵フェーズ III︶の患者登録を開始したと発表[35][36]。 2023年6月22日、厚生労働省がファビピラビルを﹁希少疾病用医薬品﹂に指定した[37]。これにより、国の助成や薬事承認審査の優遇措置が受けることができるようになり、SFTS治療薬の開発が加速することが期待されると報道された[37]。 2024年5月24日、厚生労働省の専門部会がファビピラビルをSFTSの治療薬として使用することを了承した。正式に承認されれば、世界で初めてのSFTSの治療薬となる[38]。エボラ出血熱への適用[編集]
「2014年の西アフリカエボラ出血熱流行」も参照
ドイツにおける最初のマウス実験[編集]
2002年頃から、ファビピラビルが広範囲のRNAウイルスに対して抗ウイルス効果を持つことが認識されており、インフルエンザウイルス以外のウイルスに対する効果についての研究が散発的に行われていた。
これらの結果を受け、ドイツのベルンハルト・ノホト熱帯医学研究所︵ハンブルク︶のシュテファン・ギュンター所長を中心とする研究グループは、ファビピラビルがエボラ出血熱ウイルスに対して、どの程度の効果を持つかを検証するため、2013年に同研究所のバイオセーフティーレベル4研究施設において、マウスを用いた動物実験を実施した[39]。
用いたエボラウイルスは、アメリカ疾病予防管理センターから提供を受けた野生型ザイール株︵ただし、オリジナルのZaire Mayinga 1976年株とは、わずかに2塩基対が異なっていた︶である。また、用いたマウスはI型インターフェロンαおよびβ受容体を欠く2種類のノックアウトマウス、IFNAR-/- C57BL/6およびIFNAR-/- 129/Svであり、これらは野生型ザイール株に対する致死的な感受性を持つことが他の研究で判明していた。
IFNAR-/- C57BL/6ノックアウトマウスを用いた実験は、週齢をそろえた雌のマウスを次の3つの実験群に分けて行われた。各実験群当たりのマウスは5から10匹であった。感染はエアロゾルを鼻腔から吸入させる方法で行った。
実験群1‥コントロールグループ︵対照群︶であり、ファビピラビルを投与しない
実験群2‥感染後6日目から13日目までファビピラビルを投与︵1日当たり、体重1kg当たり300mg︶
実験群3‥感染後8日目からファビピラビルを投与︵1日当たり、体重1kg当たり300mg︶
結果は次の通りであった。
実験群1‥感染から10日以内に全個体死亡
実験群2‥投与後4日︵感染後10日︶以内に血液中のウイルス消滅。感染後3週間まで全個体生存、回復
実験群3‥実験群1より若干死期を遅らせたが、感染後14日目までに全個体死亡
IFNAR-/- 129/Svノックアウトマウスを用いた実験では、予想に反してファビピラビルを投与しない対照群の生存率が80%に達したため、ファビピラビルを投与した実験群と生存率にほとんど差が出ないという結果に終わった。これは、他の研究で報告されていたのはIFNAR-/- 129/SvはザイールE718 1976年株に対して致死性を持つということであり、今回用いたZaire Mayinga 1976年株との違いによるものであろうと考えられた。
総論として、ファビピラビルはマウス実験のレベルにおいては、明らかにエボラ出血熱に対する治療効果を持つが、投薬開始が感染後6日目と8日目では生死が100%分かれるというほど、生存率は投薬開始時期に強く依存することが判明した[40]。
2014年夏以降の動き[編集]
富山化学の親会社である富士フイルムホールディングス、ならびに同社の提携先である米国のメディベクター (Medivector) 社が、アメリカ合衆国で治験を行う意向を示したことから、エボラ出血熱ウイルスに対するファビピラビルの効果について、米国および世界の関心が集まり始めた[41][42][43][44][45]。 2014年10月20日、富士フイルムは、エボラ出血熱患者への投与拡大に備え、海外におけるエボラ出血熱対策での使用を目的とした﹁アビガン錠﹂の追加生産を決定した[46]。フランスは、2014年10月21日、アビガンの臨床試験を開始すると発表した[47]。 韓国の保健福祉部は、2014年10月30日、富士フイルムとアビガンの供給について合意したと発表した。韓国でエボラ出血熱が発生した場合は、アビガンが使用されることとなった[48]。 2014年11月11日、富士フイルムホールディングスはアビガン︵ファビピラビル︶が2015年1月にもエボラ治療薬として国際承認される見通しを明らかにした[49][50][51]。承認されればエボラ治療薬第一号となる[49][50][51]。 2015年2月5日、フランス国立保健医療研究所 (INSERM) は、西アフリカ・ギニアで2014年12月17日から患者約80人に対して実施しているエボラ出血熱治療薬アビガンの臨床試験について、フランス大統領府に﹁死亡症例が減り治癒が増えている﹂と評価報告を行った。大統領府は﹁今後さらに大規模試験で確認する必要はあるが、アビガン服用はエボラとの戦いに有望と思われる﹂との声明を発表した[52]。 2015年2月24日、INSERMおよび国境なき医師団 (MSF) は、血中ウイルス量が少ない感染初期の患者の死亡率が30%から15%へと半減し有効であったが、ウイルス量の多い患者や小児では効果が得られなかったと発表した[53][54][55][56]。投与例[編集]
2014年9月26日、富士フイルムはフランスでアビガン200mg錠がエボラ出血熱ウイルスに感染したフランス人女性看護師に投与されたと発表した。これはフランス政府機関より依頼を受け、日本政府と協議の上緊急対応として提供されたものである[57][58]。この女性は10月4日、無事に回復して退院した[59][60]。 2014年10月6日、ドイツ・フランクフルト大学病院に搬送されたウガンダ人のエボラ出血熱患者の治療のために、10月4日に﹁アビガン錠﹂が投与された[61]。 2014年10月19日、スペインのエボラ対策当局は、マドリード市内の病院で患者を看護していて二次感染し、入院治療を受けていた看護助手に対してファビピラビルを投与したところ、体内からウイルスが消失したと発表した[62]。2019新型コロナウイルス[編集]
この節の加筆が望まれています。 |
2020年2月以降、COVID-19に対する薬剤転用研究の対象となっている。
しかし、2020年2月27日付けで厚生労働省が全国の保健所等に通知した事務連絡[153]でのファビラビルの処方量は過少である。 (1) 2020年2月27日付けで厚生労働省が全国の保健所等に通知した事務連絡に記載のファビピラビルの処方量での︵1日目は1,800mgを2回で3,600㎎︶は、新型コロナウイルスによる発病者の治療にアビガンを用いる場合の必要処方量としてアビガンの開発者である白木公康が提唱する1日あたり6g~9g︵6,000mg~9,000mg︶[155]の中心値である7,500mgと比較すると、48%となっている。しかし、1日あたり6g~9gを投与した臨床経験はない[156]。よって、1日あたり6g~9gを投与する新型コロナウイルス患者における検証試験を実施する場合、追加臨床試験で忍容性が確認されることが前提となる。 (2) ファビピラビルを新型コロナウイルス︵COVID-19︶の治療に用いる場合の用法・用量は、2020年2月17日付で富士フイルム富山化学工業が首相官邸に提出した資料[157]の第10ページには﹁COVID-19へのEC50濃度 9.72 μg/mL はヒト国内インフルエンザ治療時の投与量の2.5~3倍量と推定される。﹂と、記載している。この記載に基づいて、インフルエンザ治療の場合の2.5倍として必要処方量を計算すると、1日目は1回4000 mgを1日2回,2日目から5日目は1回1500 mgを1日2回経口投与する総投与期間は5日間となる。この必要処方量からみて、日本感染症学会が提唱する処方量︵1日目は1回1,800mg︵200mg錠×9錠︶を1日2回、2日目から1回800mg︵200mg錠×4錠︶を1日2回経口︶は、1日目では必要処方量の45%、2日目以降では必要処方量の53%となる。 (3) 厚生労働省が行なった事務連絡[153]でのCOVID-19へのアビガンの標準処方量︵成人向け︶は、日本小児科学会が定めたアビガンの標準処方量[158]からすると、体重が22kg~35kgの子供用のものとほぼ同じである。 (4) 添付文書に記載された新型又は再興型インフルエンザウイルス感染症での投与量は、8,000㎎︵1日目1,600mg×2 回、2~5日目600mg×2 回、総投与期間5日間︶であるが、日本感染症学会の﹁COVID-19に対する抗ウイルス薬による治療の考え方﹂[154]によると約3倍の最大24,400㎎︵1日目1,800mg×2回、2日目以降800mg×2回、最長14日間︶とされており、1回投与量及び投与期間の違いによって、備蓄量の換算人数分は異なる。[159] (5) すなわち、2020年2月27日付けで厚生労働省が全国の保健所などに通知した事務連絡[153]の添付資料となっている2020年2月26日付で日本感染症学会が発表した処方量は必要処方量のほぼ50%以下になっており、アビガンの薬効が十分には発揮できない過少なものであると思われる。
新型コロナウイルスの治療中に目が青色に変わる事例が2021年12月にインドで発生した。[168]この事例の場合は角膜が青く変色しているのが確認された。そのほかにも2023年4月ににも生後6ヶ月の男児でも目の色の変色が確認された。この男児は投与を停止してから5日目に目の色が正常に戻ったことが確認されている。そのほかにも目が蛍光を示したり爪や髪に紫外線ライトを照射した結果、蛍光が確認された事例もある。ファビピラビルを投与して体の一部が変色した事例は複数件報告されているが、詳細は明らかにはなっていない。 臨床使用における副作用等発現状況については、独立行政法人医薬品医療機器総合機構のサイトに掲載された情報によれば、インフルエンザ治療薬としての承認用法及び用量における投与実績はないが、国内臨床試験及び国際共同第III相試験︵承認用法及び用量より低用量で実施された試験︶で得られたデータは、次の通りである。 安全性評価対象症例501例中、副作用が100例︵19.96%︶に認められた︵臨床検査値異常を含む︶。主な副作用は、血中尿酸増加24例︵4.79%︶、下痢24例︵4.79%︶、好中球数減少9例︵1.80%︶、AST︵GOT︶増加9例︵1.80%︶、ALT︵GPT︶増加8例︵1.60%︶等[169]。
中国[編集]
2020年2月4日にネイチャーの姉妹誌Cell Researchに、中国科学院武漢ウイルス研究所などの研究グループが発表したレター︵速報論文︶によれば、in vitro︵試験管内︶の環境下で、アフリカミドリザル起源の標準細胞Vero E6細胞を用いて、ファビピラビルをはじめとして、エボラ出血熱治療薬として開発されたレムデシビル、抗マラリア剤のクロロキンなどを含む7種類の物質の2019新型コロナウイルス (SARS-CoV-2) に対する抗ウイルス効果を、50%効果濃度︵EC50値︶を基準として評価する試験を行ったところ以下のデータが得られた。 ●ファビピラビルのEC50値は61.88μM︵マイクロモル/リットル︶であった。 ●レムデシビルのEC50値は0.77μMであり、ファビピラビルより約80倍効果的であった。 ●クロロキンのEC50値は1.13μMと、7つの試験物質のうちではレムデシビルに次ぐ効果を示した。 しかし、エボラ出血熱ウイルスに対するファビピラビルのEC50値は67μMと、ほぼ同程度であるにもかかわらず、臨床試験・動物実験等in vivoの環境下では、エボラ出血熱ウイルスに対してはかなり良い抗ウイルス効果を示しているのに、レムデシビルは有意な効果は示していない。このため、研究グループは、ファビピラビルについてはin vivoでの試験が必要であると結論付けている[63]。 2月13日付けの中国科学技術部が発行する科技日報によれば、深圳第三人民病院で臨床研究が実施された。中程度25例、重篤例1例の計26名が対象となった。この研究では、明らかな副作用を発現せず、経過良好であり、比較的良い解熱効果を有し、服用後2日以内に72%の発熱が緩和された。また、3日以内の肺の影像学的好転率は38%で、6日間以内の肺の影像学的好転率は70%であった[64]。 2月15日付け科技日報は、上記のin vitro試験の結果に基づいて、ファビピラビル、レムデシビル、クロロキンの臨床試験開始を報じた。ファビピラビルについては70名の患者︵プラシーボを投与される対照群を含む︶で実施されており、初期の段階では明らかな治療効果と十分低い副作用を示している。投与群については、投与から3から4日後には、ウイルス核酸の排除速度は対照群に対して有意に高かった。なお、レムデシビル、クロロキンの結果については、この記事では触れられていない[65][66]。 このように、ファビピラビルがin vitro環境ではレムデシビル、クロロキンに対して大きく効果が劣るにもかかわらず、in vivoの臨床試験では著しい効果を発揮する理由について、2月17日付けの科技日報は、鍾武[67]の﹁ファビピラビルはRNAポリメラーゼ阻害剤の一種ではあるが、特殊なのは、それ自体はプロドラッグであり、RNAポリメラーゼと相互作用する競合基質として作用するために、in vivoでの三リン酸化を必要とするということである。﹂という説明を掲載している。つまり、Vero E6細胞を用いたin vitro試験では、十分な三リン酸化を受けられなかったため、本来の効果が発揮されなかったということである[68]。 浙江海正薬業股份有限公司は国家薬監局の承認を得て、ファビピラビルを正式に販売できる状態となっており、2月16日から正式に後発医薬品としての生産を開始した[69]。 3月6日付けの科技日報において、中国科学技術部生物技術開発センター所長で、中国国務院・共同予防および管理機構[70]・科学研究グループ・薬物研究チームのリーダーでもある張新民[71]は﹁深圳第三人民病院は、新冠状肺炎の治療におけるインターフェロンと組み合わせたファビピラビルの有効性と安全性に関する研究を実施し、80人の患者が登録された。その中で投与群は35例、対照群は45例であった。﹂と述べている[72]。また、張新民は﹁武漢大学中南病院は、新型コロナウイルス肺炎の治療におけるファビピラビルの多施設臨床研究を実施し、88人の患者による7日間の臨床観察を完了した。内訳は、投与群、対照群とも各々44例である。中間結果として、治療7日後の投与群の臨床回復率は対照群よりも良好であり、治療3日目の体温正常復帰率は81.8%であり、これは対照群の29.5%より有意に高く、治療6日目の咳寛解率は93.2%に達し、これは対照群の68.2%よりもかなり良い。現在、試験はまだ進行中であり、臨床的観察と結果の分析を続行中である。﹂と明らかにした[73] [74]。 3月11日に中国国務院共同予防管理機構特別薬剤研究チームが研究結果を発表した[75][76]。この研究は、軍事医学研究院・国家応急予防製薬工学技術研究センターの鍾武︵钟武、Zhong Wu、前出︶、および深圳第三人民病院の刘磊︵Liu Lei)院長、刘映霞︵Liu Yingxia)副院長を中心とするチームによって実施され、ファビピラビルを投与する患者35人と、カレトラ(ロピナビル/リトナビル)を投与する患者45人について、投与開始からウイルスが体内から排除されるまでに要した日数を比較した。結果は、ウイルス除去までに要した期間の中央値は、ファビピラビル投与群で短く、中央値(四分位範囲)は4日(2.5-9日)で、カレトラ投与群では11日(8-13日)であった。またカレトラ投与群に対してファビピラビル投与群の方が副作用は少なかった [76]。この研究結果は、刘磊、刘映霞、鍾武を責任著者︵corresponding authors︶とする公式論文 [77]にまとめられ、2020年3月18日に、中国工学院が刊行している英語版学術論文誌であるEngineeringで発表され、オランダの出版社であるエルゼビアが運営する学術論文プラットフォームサイトScienceDirectに全文(PDF形式)が掲載された。この論文では、投薬開始14日後における胸部CT影像改善率は、ファビピラビル投与群で91.4%、カレトラ投与群で62.2%としている。なお、この論文は、2020年4月8日に“TEMPORARY REMOVAL”︵一時撤回︶の状態になっていたが、4月16日に差替え版が再掲載され、再び閲覧可能な状態になっている。 3月17日、中国国務院共同予防管理機構が記者会見を開き、科学技術部・生物技術開発センター所長の張新民、工程院院士の王軍志らは、ファビピラビルが臨床研究を完了し、正式に有効性を確認したと明らかにした[78]。張新民によれば﹁安全性の観点からは新型コロナウイルス肺炎の臨床研究では、臨床的に重大な副作用は発見されていない。有効性の観点からは、深圳第三人民病院が実施した、インターフェロンと併用したファビピラビルの有効性と安全性の研究では、80人の患者が登録され、うち35人がファピラビル投与群、45人が対照群であった。 結果は、ウイルス核酸が陰性になるまでに要した時間の中央値は、投与群が対照群よりも有意に短く、それぞれ4日および11日であった。胸部X線画像の改善率に関しては、投与群と対照群でそれぞれ91.43%と62.22%であった。また、武漢大学中南病院で行われていた、前述の多施設臨床試験は、最終的に120人の患者が登録され、臨床治療観察は完了した。臨床研究の結果は、ファビピラビル投与群が新型コロナウイルス肺炎の治療において対照群よりも有意に優れていることを示している。治療終了時の中程度患者の臨床的回復率は、投与群の方が対照群よりも有意に良く、それぞれ71.43%と55.86%であった。 解熱時間に関しても、投与群は対照群よりも有意に良好であり、平均解熱時間はそれぞれ2.5日と4.2日であった。 平均咳寛解時間は、投与群よりも対照群の方が有意に長く、それぞれ4.57日と5.98日であった。治療期間中の中程度患者の補助酸素療法または機械呼吸装置の使用率は、投与群の方が対照群よりも有意に低く、それぞれ 8.16%と17.12%であった。入手可能性の観点からは、今年2月、国内企業は国家薬監局から医薬品登録の承認を取得し、大量生産を達成し、臨床薬の供給が保証されている﹂という。また、張新民は﹁ファビピラビルは医薬品の良好な安全性、明確な有効性、および入手可能性を考慮して、科学研究グループの専門家によって医療グループに正式に推奨された。できるだけ早く治療計画に組み込むよう提案する。﹂と述べた[79]。 3月23日、武漢大研究チームがファビピラビル投与で軽症者に限ると投与後7日以内の回復率が7割を超え、有効性が確認できたと発表した[80][81]。軽症例に限ると多くの症例がファビピラビル投与で4日以内に症状が消えたと報告した[80][81]。この研究は、2020年2月20日から3月12日にかけてに、武漢市の3つの病院で実施された多施設臨床研究[82]であり、ファビピラビル投与群の治療効果をUmifenovir︵商品名‥アルビドール、Arbidol︶投与群を対照群として比較評価するものであった[83]。総数240人の患者をランダムに120人ずつのグループに分け、一方をファビピラビル投与群とし他方をアルビドール投与群とした。オープン・ラベル試験であり、医師および患者には、どちらの薬が投与されるのかは開示されていた。結果は、7日目の回復率はファビピラビル投与群︵最終評価数は116人︶が71.43%であるのに対しアルビドール投与群︵最終評価数は120人︶は55.86%であり、治療効果におけるファビピラビルのアルビドールに対する優位性は、統計的に有意であった︵帰無仮説成立確率‥P=0.0199︶。日本[編集]
2020年2月22日、日本国政府は新型コロナウイルスの感染者を対象にアビガンの投与を推奨する方針を固め、富士フイルム富山化学に増産を求めた[84]。同日、加藤勝信厚生労働大臣は患者への投与を開始したことを明らかにした[85][86][87]。 3月19日に、市立札幌病院の15例報告によれば、発症11日目の60代の患者(男性)1名に対してファビピラビルの投与︵シクレソニドも併用︶が行われた[88]。ファビピラビルの適応外使用に関しては、院内臨時倫理委員会の承認を得て実施されている。投与は、発症13日目に人工心肺装置︵ECMO︶導入のため患者が他院へ転院となるまで続けられたが、この間で症状の改善はみとめられなかった[89]。また、3月26日、同病院の向井正也院長は北海道テレビ放送で記者の﹁抗HIV薬︵カレトラ︶が効いているのか?﹂という問いに対して、﹁アビガンのほうが効いていることが多い﹂と答えている [90]。 3月28日、安倍晋三首相はファビピラビルについて﹁新型コロナウイルスの治療薬として正式に承認するにあたって必要となるプロセスを開始する﹂、﹁多くの国から関心が寄せられており、希望する国々と協力しながら臨床研究を拡大し、増産をスタートする﹂と発表した[91]。 3月31日、富士フイルムは日本での治験開始を発表した[92][93]。 3月31日に発表された地域医療機能推進機構船橋中央病院の報告 [94] によれば、高齢のコロナ肺炎患者︵80代後半女性︶に対して、同院に入院した次の日からファビピラビルによる早期治療を開始したところ順調に回復し、入院16日後にPCR陰性化、18日後に退院した。観察された最も大きな副作用は尿酸値上昇であったが、これは通常の高尿酸血症治療薬︵フェブキソスタット︶の投与で解決され、大きな支障となることはなかった。他に軽い好中球数減少が認められたが、これも問題となることはなかった。他の臨床試験で観察されているASTやALT増加や下痢症状は認められなかった。 4月2日、大阪市立総合医療センターの白野倫徳医師は毎日放送で﹁︵当院では︶中等度以上の患者に使用しているが、効く人には効くが、効かない人もいる。急激に悪化する人には、いかに適切なタイミングで投与しても食い止めることはできない﹂と答えている [95]。 4月2日、日本国内で唯一のアビガンの原料を生産する設備︵新潟県糸魚川市の工場︶を有しているデンカ株式会社は、日本政府からの要請で原料を生産すると発表した[96]。 4月6日に発表された東京品川病院の報告によれば、同院に入院直後に経皮的動脈血酸素飽和度︵SpO2︶が急激に90%に低下した新型コロナウイルス患者︵39歳、男性︶に対して、急性呼吸窮迫症候群(ARDS)を防ぐ目的でファビピラビルの投与を開始したところ、翌日には解熱とSpO2の改善が認められ、投与3日目にはPCR陰性となった。同院で同様の症状で、ファビピラビルを使用せずに治療した他の新型コロナウイルス患者2例については、PCR陰性になるまで、各々27日と37日を要しており、今回の急激な改善はファビピラビルの効果による可能性が高いと考察している[97]。 4月7日、安倍首相はアビガンについて﹁120例を超える投与が行われ症状改善に効果があったと報告を受けている﹂と明らかにした[98]。 4月13日に発表された地域医療機能推進機構船橋中央病院の報告[99]では、全5例の内の4例︵20代男性、60代女性、40代男性、50代男性︶(他の1例は既報[94])のうち、どの例でもファビピラビル投与開始後は症状は悪化することは無く、遅くとも4日以内に発熱、倦怠感、食欲不振などの症状の軽快化が認められたが、うち2例では、投与期間の14日間の内にはPCR陰性化までには到らなかった。また、4例全てで大きな副作用は見られなかったとしている。 4月14日、新型コロナウイルス肺炎のため入院中のグラビアアイドルでタレントのソラ豆琴美が、Twitterで13日からアビガン投薬治療を開始したことと、病状の変化について﹁昨晩から飲み始めて早速効果があるように感じました。高熱が微熱になって、全くわからなかった味がほんの少し、味の雰囲気がわかるようになり、匂いも全くなかったのがほんのりわかりかけた気がします。咳の量も減って、痰︵たん︶もかなり減りました。まだ1日目なので劇的な変化ではないけど明らかにいい感じ!﹂[100] と報告している。効果が投薬開始後1日程度で急速に現れるという点で、東京品川病院での症例[97](前述)と共通する面がある。 4月18日に開催された日本感染症学会の緊急シンポジウムにおいて、藤田医科大学︵愛知県豊明市︶の土井洋平教授は、同大が主導して実施しているファビピラビルの国内第Ⅲ相臨床試験の状況について報告し、ファビピラビルを投与された300人のうち、軽症と中等症の患者ではおよそ9割、人工呼吸器が必要な重症患者では6割で2週間後に症状の改善が見られたことを明らかにした[101]。この9割改善という数値は、中国の刘磊、刘映霞、鍾武らによる論文[77]における数値、および後述のイランの医師の主張︵30人中27人回復︶と一致している。 4月19日、西村康稔新型コロナウイルス感染症対策担当大臣は、ファビピラビルについて、700を超える医療機関・施設で手続きを終えて、患者の同意があれば使えるようになったと述べた[102]。 4月21日、加藤勝信厚生労働大臣は、現在、治療薬の審査期間を6か月程度を目標に短縮する﹁先駆け審査指定制度﹂という制度があるが、アビガンなど新型コロナウイルス治療薬の承認審査については、﹁6か月にこだわるつもりは全くなく、出来る限り短く対応していきたい﹂と述べた[103]。 同日、赤司浩一九州大学病院長、岩崎昭憲福岡大学病院長、高島宗一郎福岡市長は、投与の承認手続きを省略して医師の判断でアビガンの投与が可能となるよう規制緩和を求める要望書を、加藤厚生労働大臣に提出した[104]。 4月27日、厚生労働省新型コロナウイルス感染症対策推進本部は、ファビピラビル使用における医療機関向けの注意事項をまとめた文書﹁コロナウイルス感染症に対するアビガン︵一般名‥ファビピラビル︶に係る観察研究の概要及び同研究に使用するための医薬品の提供について﹂[105] を公表した。これによれば、医療機関が同剤を投与する条件としては、倫理委員会などで承認を得たうえで、藤田医科大学病院などの研究班が行っている観察研究に参加し、患者本人の同意があり、医師の判断によって使用が必要となった場合に限り可能であるとしている。同剤は治療薬としては承認されていないが、既に全国の医療機関で観察研究として広く投与が行われており、参考情報として、4月26日の時点で、既に国内1,100の医療機関で、2,194人の患者に投与されたと述べている。なお、4月26日の時点で国内感染者は13,232名、そのうち入院患者は8,051名であり、入院患者のうちの約4分の1は同剤の投与を受けていることになる。 5月4日、安倍首相は記者会見で、アビガンが臨床研究で有効性が確認されれば5月中の承認を目指す考えを示した[106]。 5月11日、福岡県医師会は、アビガンの観察研究への医療機関の参加を、医師会が設けた倫理委員会が一括して審査する﹁福岡県方式﹂を、観察研究を実施している藤田医科大学と厚生労働省に提案し、承諾を得たと発表した[107]。同剤の投与は、観察研究への参加という形で、現在既に全国で実施されているが、観察研究への参加に際しては、医療機関ごとに設置する倫理委員会などで承認を得る必要があった。福岡県方式は、この手続きを簡略化するものであり、観察研究による投与の枠組み自体を変えるものではないが、今後は医師会に登録した医療機関で、医師の判断と患者の同意があれば投与が可能になり、軽症者への早期投与が可能となった。ただし、副作用の管理対策として当面は入院患者に限定される。 特定臨床研究﹁SARS-CoV2感染無症状・軽症患者におけるウイルス量低減効果の検討を目的としたファビピラビルの多施設非盲検ランダム化臨床試験﹂の中間解析で、顕著な有効性を確認できることを期待して、﹁新型コロナウイルス感染症に対する医薬品等の承認審査上の取扱いについて︵薬生薬審発 0512 第4号、薬生機審発 0512 第1号︶﹂︵令和2年5月12日︶を発出したが、目論見が外れた[108][109]。 5月26日付けの毎日新聞、日本経済新聞及び朝日新聞は、加藤勝信厚生労働相は同日午前の記者会見で、﹁臨床研究の継続の可否を判断する中間解析において、極めて高い有効性が示されればその結果を待つまでもなく、薬事承認という流れも想定していた﹂とするが、学外の専門家による評価委員会から科学的に評価することは時期尚早とされたため研究は継続となり、月内の承認には至らなかった[110][111][112]、と報道しているが、その前日の5月25日付けの産経新聞など複数のメディアは、﹁25日時点で審査の前提となる企業からの承認申請はなく、月内に審査を終えるのは不可能と判断した。政府関係者が明らかにした。﹂このため﹁安倍晋三首相が目指すとした﹃5月中の承認﹄を政府が断念したことが25日、分かった。﹂[113]と、承認できないのは企業側の未申請が原因であると報道しており、報道に齟齬が見られる。 5月26日に発表された藤田医科大学の﹁︻中間報告︼ファビピラビル観察研究中間報告︵2020年5月15日現在︶﹂[114]によれば、5月15日の時点でファビピラビルの観察研究による投与例が、全国407の医療施設から2158例登録されている。このうち軽症患者が976名︵45.2%︶、中等症患者が947名︵43.9%︶、重症患者が235名︵10.9%︶であり、コロナ肺炎の発症者の8割は軽症のまま治ることがわかっているので[115]、観察群の構成は通常よりは中等症患者と重症患者の比率が高い︵54.8%︶。投与開始後7日目の改善率は、軽症が73.8%、中等症が66.6%、重症が40.1%︵サンプル数1,713︶であり、投与開始後14日目の改善率は、軽症が87.8%、中等症が84.5%、重症が60.3%︵サンプル数1,282︶であった。また、入院後1ヶ月の時点における死亡率は、軽症が5.1%、中等症が12.7%、重症が31.7%、全体で11.6%︵サンプル数1,918︶であった。副作用については、2,158名のうち、尿酸値上昇または高尿酸血症15.58%、肝障害または肝機能酵素上昇7.37%が報告された。考察として、対照試験ではないこと、前述のように発症者の8割は軽症のまま治ることから、﹁慎重に結果を解釈することが必要﹂として、有効との結論は控えている。 7月10日、特定臨床研究﹁SARS-CoV2感染無症状・軽症患者におけるウイルス量低減効果の検討を目的としたファビピラビルの多施設非盲検ランダム化臨床試験﹂の最終結果の暫定的な解析が報告された[116]。目標症例数は、86名で、89名が登録され、ランダム化された。主要評価項目である﹁6日目まで︵遅延投与群が内服を開始するまで︶の累積ウイルス消失率﹂は、通常投与群で66.7%、遅延投与群で56.1%、調整後ハザード比は1.42︵95%信頼区間=0.76-2.62、P値=0.269︶であった。副次評価項目である﹁6日目までのウイルス量対数値50%減少割合﹂は通常投与群で94.4%、遅延投与群で78.8%、調整後オッズ比は4.75︵95%信頼区間=0.88-25.76、P値=0.071︶であった。主要評価項目及び副次評価項目ともに、統計学的に有意な差は認められなかった。 9月10日に全国医学部長病院長会議(AJMC)が発表した、﹁新型コロナウイルス感染症における重症症例に対する治療実態調査結果﹂[117]では、AJMC会員である全82国公私立大学病院から得られた回答を集計したところ、7月31日までに治療を行った487例の重症症例のうち、378例(77.62%)でアビガンが使用されたことが明らかになった。レムデシビルの使用例は54例(11.09%)であった。なお、重症症例487例のうち死亡は98例(20.1%)であり、このうちアビガンを使用した378例では死亡は74例(19.58%)であり、アビガンを使用しなかった109例では死亡は24例(22.02%)であった。 9月23日、新型コロナウイルス感染症患者を対象としたアビガンの国内臨床第Ⅲ相試験結果が、富士フイルムホールディングスのニュースリリースで報告された[118]。156例を解析対象とした主要評価項目︵症状︵体温、酸素飽和度、胸部画像︶の軽快かつウイルスの陰性化までの時間︶の中央値は、アビガン投与群で11.9日、プラセボ投与群では14.7日となり、非重篤な肺炎を有するCOVID-19患者にアビガンを投与することで早期に症状を改善することを、統計学的有意差︵p値=0.0136︶をもって確認した。調整後ハザード比は、1.593 (95%信頼区間1.024 – 2.479) を示した。さらに本試験では、安全性上の新たな懸念は認められなかった。 10月16日に富士フイルムホールディングスが、抗インフルエンザウイルス薬﹁アビガン®錠﹂について富士フイルム富山化学株式会社が新型コロナウイルス感染症に係る効能・効果などを追加する製造販売承認事項一部変更承認申請を厚生労働省に行ったことを発表した[119][120]。 12月21日、厚生労働省は、薬事・食品衛生審議会の専門部会を開き、アビガンを新型コロナウイルス感染症の治療薬として承認するかを審議した。富士フイルム富山化学による治験が、どの患者にアビガンを投与したかを医師が把握して行われる単盲検試験だったことの影響、治験結果の臨床的意義について議論した。現時点では﹁有効性を明確に判断することは困難﹂とされ、継続審議となった。海外で実施中の臨床試験︵治験︶などの結果の提出を待ち、年明け以降に審議する[121][122]。 2021年2月21日、富士フイルムホールディングスは、アビガンについて、4月にも国内で臨床試験︵治験︶を再び実施する方針を固めたと報じられた[123][124]。既実施治験が、単盲検試験だったことで、﹁有効性を明確に判断することは困難﹂とされたことから、再試験では、二重盲検試験とする。対象は、65歳以上の軽症患者、腎疾患、糖尿病等の基礎疾患を有する50歳以上の軽症患者で、目標症例数は、約270名としている。 4月21日、富士フイルムホールディングスが、抗インフルエンザウイルス薬﹁アビガン®錠﹂について、富士フイルム富山化学株式会社が新型コロナウイルス感染症の患者を対象とした新たな第3相試験を国内で開始したことを発表した[125]。発熱などの症状発現から72時間以内、かつ基礎疾患や肥満などの重症化リスク因子を有する、50歳以上のCOVID-19患者において、重症化した患者の割合を主要評価項目とし、有効性を検証する二重盲検プラセボ対照試験である。 2022年3月11日、富士フイルムホールディングスが、アビガンの国内臨床試験︵治験︶で新規投与を終了すると発表した。従来の変異型と比べて重症化率が低いオミクロン株の流行により、アビガンが重症化を防いだかという検証が難しくなっている上に、治験の条件となるワクチン未接種者も減っていることも要因とされている[126]。アメリカ[編集]
2020年4月9日、富士フイルムはアメリカでのアビガンの治験を第2相試験に進めると発表[127][12]。第2相臨床試験は2020年6月末までに終える予定である[12]。米国ではファビピラビルは未承認であり、米国で承認申請を行うには第3相臨床試験で有効性を確認することが必要になる[12]。 12月2日、Appili Therapeutics Inc.は、アビガンのアメリカでの第3相試験において、最初の患者へ投与したことを発表した[128]。本試験は、ランダム化プラセボ対照二重盲検比較試験であり、軽症及び中等症の外来患者が対象、目標症例数は、826名である[129]。 2021年11月12日、Appili Therapeutics Inc.は、アビガンのアメリカでの第3相試験で、主要評価項目である持続的な臨床回復までの期間について、統計学的有意差を達成しなかったことを発表した[130]。試験には、米国、メキシコ、ブラジルの38の治験施設からCOVID-19患者1,231人が登録された。イラン[編集]
2020年3月20日、イランの政府系通信社タスニム通信は、イラン新年初日︵3月20日︶に茂木敏充外務大臣とイランのザリフ外相が電話会談を行い、茂木大臣は日本政府はイランに対して無償でファビピラビルを供与する予定であると述べたと報道した [131]。 4月3日、イラン保健省のキアヌーシュ・ジャハンプール︵Kianoush Jahanpour︶報道官は、ファビピラビルに関して﹁︵中国で実施された臨床試験について︶患者に相当な効果を与えているが、効果を科学的に立証したとまで言うには不十分であろう﹂﹁日本から寄付された薬の臨床試験の結果はまだ出ていない﹂﹁既に日本からは3,000人分の薬を寄付を受け、さらに中国からは中国赤十字(中国紅十字会)を通じて15,000人分の薬を寄付されている。これらは多数の病院で臨床試験中であり、現在、研究と効果評価を行っている最中である﹂と発表した[132]。 4月5日付けのタスニム通信によれば、テヘランの国営マシ・デーンズバリ病院︵Masih Daneshvari hospital︶のヴェラーヤティー院長は、シャヒード・ベヘシュティ―大学が、イラン国内では初めてアビガンの製造に成功したと発表した。同院長によれば、製造された薬は既に同病院でのコロナ肺炎患者の治療に用いられている。なお、ヴェラーヤティー院長自身もコロナ肺炎に罹患し、回復している[133]。 同日、イラン国外を拠点とするイラン反体制派組織イラン国民抵抗評議会は、上記のマシ・デーンズバリ病院の医師 Mohammad Reza Hashemian が、﹁この薬により30人のコロナ肺炎患者の内、27人が劇的に回復しているにもかかわらず、イラン保健省は情報を隠蔽し、国民が使用するのを妨害している﹂と非難していると伝えている。同医師は、政府の意図について、薬を体制側の人間に優先して使用するための数の確保が目的であり、実際、コロナ肺炎に罹患した、体制側の重要人物であるヴェラーヤティー院長は、この薬を優先的に割り当てられたおかげで回復したと主張している[134]。イタリア[編集]
2020年3月22日、イタリア医薬品庁︵アイファ、AIFA︶は、同庁の科学技術委員会は、最も新型コロナウイルス感染症の拡大が激しい3つの地域、ロンバルディア、ベネト、エミリアロマーニャの病院におけるファビピラビルの試験的投与を承認したと発表した[135]。ただし、イタリア医薬品庁は﹁この薬はヨーロッパでもアメリカでも認可されていない。 この薬物の効果について現在ある証拠は、まだ不十分で予備的なものである﹂と釘を刺している[136][137]。トルコ[編集]
2020年3月23日に、コジャ保健相は、中国から供与された本薬の患者への投与を既に実施していると発表した[138][注 2]。トルコ国営・アナドル通信社によれば、この治療薬は国内の40の都市に空輸された。 6月9日、エルドアン大統領は、トルコ国内でファビピラビルの製造を行う予定であり、登録手続きが完了すればコロナ肺炎治療に使用可能になると述べた。既に、中国からトルコに持ち込まれたファビピラビルを用いて行われた治験では、治療期間の短縮と、肺への病状の改善の両方において効果が確認されている[140]。ドイツ[編集]
2020年4月、ドイツ政府がアビガン数百万セットを大量購入すると報道された[141][142]。韓国[編集]
2020年3月16日付け中央日報によれば、韓国政府はアビガンの輸入特例を検討していたが、中央臨床委員会など専門家らの意見に従い、本薬の効果や副作用の強さなどから臨床的根拠が不十分であるため、導入しないと発表した。﹃ネイチャー﹄に掲載された中国の研究論文を分析した結果、アビガンが新型コロナウイルス抑制効果がなく、副作用も深刻であり使用できないとした[143]。 中央臨床委員会のオ・ミョンドン委員長︵ソウル大病院感染内科教授︶はアビガンが中国で治療薬として許可されたと報道されたが、﹁ネイチャーの論文によると﹃単に臨床試験患者を募集する﹄と話しただけ﹂であり、またWHOも治療薬候補にアビガンを挙げていないとして、アビガンが治療薬として許可されたというのはフェイクニュースだと論じた[143]。イギリス[編集]
2020年5月1日、ロンドンのチェルシー・アンド・ウェストミンスター病院は、450名の患者が参加するファビピラビルとヒドロキシクロロキンの臨床試験を開始すると発表した。試験では患者を150人ずつの3つのグループに分け、第1グループにはファビピラビルを投与し、第2グループにはヒドロキシクロロキン、亜鉛およびアジスロマイシンを投与し、第3グループは対照群として通常の治療法による処置のみを行う。また、同病院の他に複数の病院も試験に加わる予定であると述べている[144][145]。ロシア[編集]
2020年5月31日、ロシアの政府系ファンドであるロシア直接投資基金(RDIF)は、同基金が合弁事業で開発したファビピラビルのジェネリック医薬品である﹁アビファビル﹂(Avifavir)に、ロシア保健省が暫定承認を与えたと発表した[146]。これはファビピラビルのコロナ肺炎治療薬としての政府機関による世界初の承認となる[147]。ChemRarは5月13日に、ロシアで4月から開始されていたアビファビルの治験で、有効性が確認できたと発表していた。治験の中間解析の結果、ファビピラビルを投与された40名のうち60%が投与開始から5日目の検査で陰性となり、標準治療群の2倍だったと明らかにしていた[148]。6月11日から、一般病院での投与が順次開始されるという。インド[編集]
2020年5月12日、Glenmarkは、ジェネリックであるファビピラビル︵販売名‥FabiFlu®︶の第3相ランダム化比較臨床試験の開始を発表した。150人の軽症から中等症患者が、標準的支持療法群あるいは標準的支持療法+ファビピラビル群に、1‥1の比率で割り付けられる[149]。 2020年5月21日、Strides Pharma Scienceは、先発薬であるアビガンとの生物学的同等性試験の実施について、インド医薬品管理局からの承認を得たと発表した[150]。 2020年5月26日、Glenmarkは、ファビピラビルのUmifenovirとの併用による第3相ランダム化比較臨床試験の実施について、インドの規制当局からの承認を得たと発表した。入院している158人の中等症患者が、ファビピラビル群あるいはファビピラビル+Umifenovir併用群に割り付けられる[151]。 2020年6月20日、Glenmarkは、ジェネリックであるファビピラビル︵販売名‥FabiFlu®︶について、インドの規制当局から軽症から中等症患者を適応として﹁制限された緊急使用﹂の製造販売承認を取得したと発表した[152]。 2020年7月24日、Cipla Ltdは、ジェネリックであるファビピラビル︵販売名‥Ciplenza︶について、インドの規制当局から製造販売承認を取得したと発表した[25]。用法[編集]
通常、成人に1日目は1回1,600 mgを1日2回、2-5日目は1回600 mgを1日2回経口投与する。総投与期間は5日間まで[7]。アビガン錠は200 mgなので、一人当たりの投与量は (1600 × 2 + 600 × 2 × 4) mg / 200 mg = 40錠となる。研究では高用量投与︵ハイドーズ︶も行われている。2019新型コロナウイルス臨床試験での用法[編集]
●中国・深圳第三人民病院での実施例[76]では、成人に対して、経口投与により、1日目は3,200mg、2日目から14日目は1日あたり1,200mg。投与期間は、ウイルスがなくなるまで、または最長14日間。 ●2020年2月27日付けで厚生労働省が全国の保健所などに通知した事務連絡[153]の添付資料となっている2020年2月26日付で日本感染症学会が発表した方針[154]によれば、1日目は1回1,800mg︵200mg錠×9錠︶を1日2回、2日目から1回800mg︵200mg錠×4錠︶を1日2回経口、最長14日間投与︵上記の中国における用法よりやや多い︶。総投与量は24,400mg︵200mg錠×122錠︶となる。しかし、2020年2月27日付けで厚生労働省が全国の保健所等に通知した事務連絡[153]でのファビラビルの処方量は過少である。 (1) 2020年2月27日付けで厚生労働省が全国の保健所等に通知した事務連絡に記載のファビピラビルの処方量での︵1日目は1,800mgを2回で3,600㎎︶は、新型コロナウイルスによる発病者の治療にアビガンを用いる場合の必要処方量としてアビガンの開発者である白木公康が提唱する1日あたり6g~9g︵6,000mg~9,000mg︶[155]の中心値である7,500mgと比較すると、48%となっている。しかし、1日あたり6g~9gを投与した臨床経験はない[156]。よって、1日あたり6g~9gを投与する新型コロナウイルス患者における検証試験を実施する場合、追加臨床試験で忍容性が確認されることが前提となる。 (2) ファビピラビルを新型コロナウイルス︵COVID-19︶の治療に用いる場合の用法・用量は、2020年2月17日付で富士フイルム富山化学工業が首相官邸に提出した資料[157]の第10ページには﹁COVID-19へのEC50濃度 9.72 μg/mL はヒト国内インフルエンザ治療時の投与量の2.5~3倍量と推定される。﹂と、記載している。この記載に基づいて、インフルエンザ治療の場合の2.5倍として必要処方量を計算すると、1日目は1回4000 mgを1日2回,2日目から5日目は1回1500 mgを1日2回経口投与する総投与期間は5日間となる。この必要処方量からみて、日本感染症学会が提唱する処方量︵1日目は1回1,800mg︵200mg錠×9錠︶を1日2回、2日目から1回800mg︵200mg錠×4錠︶を1日2回経口︶は、1日目では必要処方量の45%、2日目以降では必要処方量の53%となる。 (3) 厚生労働省が行なった事務連絡[153]でのCOVID-19へのアビガンの標準処方量︵成人向け︶は、日本小児科学会が定めたアビガンの標準処方量[158]からすると、体重が22kg~35kgの子供用のものとほぼ同じである。 (4) 添付文書に記載された新型又は再興型インフルエンザウイルス感染症での投与量は、8,000㎎︵1日目1,600mg×2 回、2~5日目600mg×2 回、総投与期間5日間︶であるが、日本感染症学会の﹁COVID-19に対する抗ウイルス薬による治療の考え方﹂[154]によると約3倍の最大24,400㎎︵1日目1,800mg×2回、2日目以降800mg×2回、最長14日間︶とされており、1回投与量及び投与期間の違いによって、備蓄量の換算人数分は異なる。[159] (5) すなわち、2020年2月27日付けで厚生労働省が全国の保健所などに通知した事務連絡[153]の添付資料となっている2020年2月26日付で日本感染症学会が発表した処方量は必要処方量のほぼ50%以下になっており、アビガンの薬効が十分には発揮できない過少なものであると思われる。
2020年2月27日付けで厚生労働省が全国の保健所等に通知した事務連絡の影響[編集]
2020年2月27日付けで厚生労働省が全国の保健所等に通知した事務連絡[153]に添付された日本感染症学会の文書に記載の処方量が過少である結果、上記[154]の処方量︵1日目は1,800mgを2回で3,600㎎︶で実施していた観察研究について、2020年5月26日に発表された藤田医科大学の﹁︻中間報告︼ファビピラビル観察研究中間報告︵2020年5月15日現在︶﹂[114]では、アビガンの有効性を明確には示すことができずに終わったと思われる。[160] この観察研究では、1日目のみ1回1,800mg×2回、2日目以降は1回800mg×2回で投与された患者が92.8%、1日目のみ1回1,600mg×2回、2日目以降は1回600mg×2回で投与された患者が5.4%、その他が1.9%である[114]。すなわち、ほとんどの患者が、2020年2月17日付で富士フイルム富山化学工業が首相官邸に提出した資料[157]に記載の﹁ヒト国内インフルエンザ治療時の投与量の2.5~3倍﹂ではなく、2020年2月27日付けで厚生労働省が全国の保健所等に通知した事務連絡[153]でのファビラビルの処方量を投与された。この観察研究でアビガンの有効性を結論しなかったのは、用法用量の違いだけが理由ではなく、観察研究の報告書に記載されているように、対照試験ではないこと、発症者の8割は軽症のまま治ることから、﹁慎重に結果を解釈することが必要﹂という状況となったためである[114]。 富士フイルム富山化学が、新型コロナウイルス感染症︵COVID-19︶について実施していた﹁アビガン﹂︵ファビピラビル︶の第3相臨床試験︵国内の臨床試験のデータベースの登録番号‥JapicCTI-205238︶での用法・用量は、1日目のみ1回1,800mg×2回、2日目以降は1回800mg×2回で、最長14日間、経口投与するものであった。[161] すなわち、この治験での用法用量は2020年2月17日付で富士フイルム富山化学工業が首相官邸に提出した資料[157]に記載の﹁ヒト国内インフルエンザ治療時の投与量の2.5~3倍﹂の約50%となっている。この過少な用量での治験の結果、156人の肺炎患者においてアビガンを投与した患者と偽薬を投与した患者ではウイルスが陰性になるまでの日数の中央値が、アビガンを投与した患者は11.9日で、偽薬では14.7日であった。[162]プラセボを対照としたアビガンの主要評価項目の統計学的有意差︵p値=0.0136︶を検証、アビガンを投与することで症状が早期に改善することを確認したことから、富士フイルムホールディングスは、10月にも新型コロナウイルスの治療薬として、アビガンの製造販売の承認を申請すると発表した[163][164][165]。副作用・禁忌[編集]
妊娠した動物への実験で催奇形性が確認されているため、妊婦および避妊無し性交渉から2週間未満の女性への投与は禁忌である。[1][166][167]。また上記の理由のために男女とも薬が体内に残っている投与期間中および投与終了後7日間、性交を行う場合は必ず避妊しなければならない[1][166][167]。新型コロナウイルスの治療中に目が青色に変わる事例が2021年12月にインドで発生した。[168]この事例の場合は角膜が青く変色しているのが確認された。そのほかにも2023年4月ににも生後6ヶ月の男児でも目の色の変色が確認された。この男児は投与を停止してから5日目に目の色が正常に戻ったことが確認されている。そのほかにも目が蛍光を示したり爪や髪に紫外線ライトを照射した結果、蛍光が確認された事例もある。ファビピラビルを投与して体の一部が変色した事例は複数件報告されているが、詳細は明らかにはなっていない。 臨床使用における副作用等発現状況については、独立行政法人医薬品医療機器総合機構のサイトに掲載された情報によれば、インフルエンザ治療薬としての承認用法及び用量における投与実績はないが、国内臨床試験及び国際共同第III相試験︵承認用法及び用量より低用量で実施された試験︶で得られたデータは、次の通りである。 安全性評価対象症例501例中、副作用が100例︵19.96%︶に認められた︵臨床検査値異常を含む︶。主な副作用は、血中尿酸増加24例︵4.79%︶、下痢24例︵4.79%︶、好中球数減少9例︵1.80%︶、AST︵GOT︶増加9例︵1.80%︶、ALT︵GPT︶増加8例︵1.60%︶等[169]。
受賞[編集]
平成30年度科学技術分野の文部科学大臣表彰﹁科学技術賞﹂を受賞[170][171]。脚注[編集]
注釈[編集]
出典[編集]
(一)^ abcd“エボラ出血熱で世界が注目する日本発のある薬剤”. 日経メディカル. (2014年8月11日) 2014年8月18日閲覧。
(二)^ [1]
(三)^ “富士フイルム、インフル薬﹁アビガン﹂のライセンスを中国大手に”. 日本経済新聞 (日本経済新聞社). (2016年6月23日) 2020年4月17日閲覧。
(四)^ abcd久保田 文 (2020年3月18日). “中国企業製造のファビピラビル、﹁COVID-19に有効﹂と中国科技部”. 日経バイオテクノロジー (日経BP) 2020年4月17日閲覧。
(五)^ “Favipiravir (T-705), a novel viral RNA polymerase inhibitor”. Antiviral Research 100 (2): 446–54. (November 2013). doi:10.1016/j.antiviral.2013.09.015. PMC 3880838. PMID 24084488.
(六)^ 白木公康 (2020年3月25日). “緊急寄稿︵2︶新型コロナウイルス感染症︵COVID-19︶治療候補薬アビガンの特徴”. 日本医事新報社. 2020年4月24日閲覧。
(七)^ abc抗インフルエンザウイルス薬﹁アビガン錠200mg﹂の日本国内での製造販売承認取得のお知らせ - 富山化学工業・2014年3月24日
(八)^ Favipiravir elicits antiviral mutagenesis during virus replication in vivo eLife
(九)^ “ノロウイルスにも効果か 日本のインフル薬、英研究”. 47NEWS. (2014年10月22日) 2014年10月22日閲覧。
(十)^ “Flu drug aimed at Ebola may also fight norovirus, study finds”. Reuters. (2014年10月21日) 2014年10月22日閲覧。
(11)^ “エボラ出血熱への効果が期待される﹁アビガン錠﹂、ノロウイルスにも効果か”. マイナビニュース. (2014年10月22日) 2014年10月25日閲覧。
(12)^ abcd富士フイルム、日本に次ぎ米国で新型コロナに﹁アビガン﹂の臨床試験へ日経バイオテク2020.04.10久保田文
(13)^ “加藤厚労相 新型コロナ感染患者にアビガン投与へ レムデジビルの効果検証も”. ミクスonline. (2020年2月25日) 2020年2月26日閲覧。
(14)^ ab条件付き承認で普及に足かせ 富山化学インフル薬の“無念” 週刊ダイヤモンド 2014年2月25日
(15)^ “ゾフルーザ採用見送り”. 亀田メディカルセンター 亀田総合病院 感染症科 (2018年11月11日). 2020年2月26日閲覧。
(16)^ “新型インフル備えアビガン錠3万人分備蓄へ 厚労省、今月中にも富山化学工業と契約”. CBNews (2017年3月9日). 2017年3月10日閲覧。
(17)^ “︻新型インフル有識者会議︼アビガン備蓄、政府指針に‐約200万人分を上限目標”. 薬事日報 (2017年4月5日). 2020年2月26日閲覧。
(18)^ ︻通知︼新型インフルエンザ発生時の国が備蓄しているファビピラビルの放出方法について︵2018年︵平成30年︶3月1日、健感発0313第1号、薬生薬審発0313第1号、薬生安発0313第1号︶ (PDF) 富士フイルム富山化学株式会社
(19)^ ︻別添1︼ファビピラビル︵アビガン︶の流通体制 (PDF) 富士フイルム富山化学株式会社
(20)^ “中国での﹁アビガン有効﹂を喜べない富士フイルム”. 日経ビジネス. (2020年3月19日) 2020年3月19日閲覧。
(21)^ “新型コロナの有望薬﹁アビガン﹂﹁レムデシビル﹂ってどんな薬?”. 日経ビジネス. (2020年2月27日) 2020年4月20日閲覧。
(22)^ “落札者等の公示 抗インフルエンザウイルス薬︵アビガン錠200mg︶約3万人分の購入”. 官報︵日本国政府︶. (2017年6月1日) 2020年4月20日閲覧。
(23)^ “Glenmark launches Favipiravir: Medics say 'no magic bullet' for COVID-19 amid rising cases”. The New Indian Express︵2020年6月20日︶. 2020年7月3日閲覧。
(24)^ “Jenburkt Pharmaceuticals second in India to launch Favipiravir (FaviventTM) to fight COVID-19”. Express Pharma (2020年7月24日). 2020年7月29日閲覧。
(25)^ ab“Cipla gets India approval to sell COVID-19 drug favipiravir”. Reuters. (2020年7月24日) 2020年7月29日閲覧。
(26)^ “Coronavirus treatment | Sun Pharma launches Favipiravir at Rs 35 per tablet”. Moneycontrol(2020年8月4日). 2020年8月4日閲覧。
(27)^ 中國H7N9變種抗藥 恐無藥醫 ︵繁体字中国語︶ 自由時報 2017年2月21日
(28)^ 男染H7N9變種 疾管署首見急釋﹁伊波拉解藥﹂ NOWnews 今日新聞 2017年2月21日
(29)^ 鳥インフルエンザウイルス H7N9型に感染で初の台湾人死者 LINE Corporation/中央社フォーカス台湾 2017年2月28日
(30)^ ab“インフル治療薬アビガン、マダニ感染症に有効 厚労省研究班”. 日本経済新聞. (2016年2月22日) 2016年11月3日閲覧。
(31)^ “マダニ感染症患者にアビガンを投与 愛媛大など臨床研究”. 朝日新聞. (2016年6月22日) 2016年11月3日閲覧。
(32)^ “インフル薬﹁アビガン﹂、マダニ感染症の臨床試験開始”. 日本経済新聞. (2016年6月14日) 2016年11月3日閲覧。
(33)^ “マダニ感染症有効か、インフル治療薬投与へ 約30の医療機関、臨床研究に着手”. 毎日新聞. (2016年6月19日) 2016年11月3日閲覧。
(34)^ ab“抗インフル薬、マダニ感染症に効果 治療法確立を目指す”. 朝日新聞. (2017年11月9日) 2018年1月14日閲覧。
(35)^ “富山化学、マダニ媒介性感染症のSFTSを対象とした抗ウイルス薬﹁ファビピラビル﹂の国内臨床第III相試験の患者登録を開始”. 日本経済新聞 電子版. 日本経済新聞社 (2018年3月12日). 2020年3月13日閲覧。
(36)^ “富山化学 抗ウイルス薬アビガン、マダニによる重症熱性血小板減少症候群でフェーズ3”. www.mixonline.jp. 株式会社ミクス (MIX, Inc.) (2018年3月13日). 2020年3月13日閲覧。
(37)^ ab“アビガン﹁希少疾病用﹂指定 厚労省、マダニ感染症効果 富山化学開発|社会|富山のニュース|富山新聞”. 富山新聞. 富山新聞社 (2023年6月28日). 2023年9月28日閲覧。
(38)^ “﹁アビガン﹂をマダニ媒介の感染症﹁SFTS﹂の世界初治療薬へ 厚労省の専門部会が了承”. TBS NEWS DIG Powered by JNN. TBSテレビ (2024年5月24日). 2024年5月24日閲覧。
(39)^ エボラ出血熱 治療法の研究本格化 - 産経新聞・2014年4月7日
(40)^ Gunther et al., February 2014, Successful treatment of advanced Ebola virus infection with T-705 (favipiravir) in a small animal model
(41)^ 富士フイルム株が急騰、年初来高値 エボラ出血熱に同社インフル薬有望で - J-CASTニュース・2014年8月8日
(42)^ “米、エボラ熱の新薬実用化急ぐ 富士フイルムなど優先審査”. 日本経済新聞. (2014年8月8日) 2014年8月13日閲覧。
(43)^ “富士フイルムのインフル治験薬、エボラ出血熱治療に有望か”. ブルームバーグ. (2014年8月7日). オリジナルの2014年8月12日時点におけるアーカイブ。 2014年8月13日閲覧。
(44)^ “エボラ出血熱﹁国際的緊急事態﹂富士フイルム“特効薬”治療利用”. スポーツ報知. (2014年8月9日). オリジナルの2014年8月12日時点におけるアーカイブ。 2014年8月13日閲覧。
(45)^ “エボラ熱治療薬候補に 富士フイルムの薬 米で手続き”. (2014年8月8日) 2014年8月13日閲覧。
(46)^ ﹁アビガン錠200mg﹂のエボラ出血熱向け生産について
(47)^ 三井美奈 (2014年10月21日). “フランス、日本のエボラ未承認薬を臨床試験へ”. 読売新聞 2014年10月21日閲覧。
(48)^ “韓国、エボラ感染発生した場合には日本治療剤を緊急導入”. 中央日報. (2014年10月30日) 2014年11月1日閲覧。
(49)^ ab“アビガン、エボラ治療薬として年明けにも国際承認=富士フイルム”. ロイター. (2014年11月11日) 2014年11月11日閲覧。
(50)^ ab“富士フイルムHD:アビガン、エボラ薬1号 1月にも承認”. 毎日新聞. (2014年11月11日) 2014年11月11日閲覧。
(51)^ ab“エボラ熱に効く薬﹁アビガン﹂が年明けにもフランスなどで承認へ 富士フイルムが見通し公表”. 産経新聞. (2014年11月11日) 2014年11月11日閲覧。
(52)^ “日本薬がエボラ熱治療に﹁有望﹂ 仏機関が臨床試験”. 共同通信 (2015年2月5日). 2015年2月6日閲覧。
(53)^ “エボラ出血熱‥ファビピラビル臨床試験の初期成績は﹁一部の患者に有効﹂”. 国境なき医師団 (2015年2月24日). 2015年2月26日閲覧。
(54)^ “アビガン臨床試験、一部でエボラ死亡率半減も”. 読売新聞. (2015年2月25日) 2015年2月27日閲覧。
(55)^ “エボラ出血熱‥ファビピラビル臨床試験の初期成績は﹁一部の患者に有効﹂”. 産経新聞. (2015年2月24日) 2015年2月27日閲覧。
(56)^ “Preliminary results of the JIKI clinical trial to test the efficacy of favipiravir in reducing mortality in individuals infected by Ebola virus in Guinea.”. MSF (2015年2月24日). 2015年4月4日閲覧。
(57)^ ﹁アビガン錠200mg﹂のエボラ出血熱ウイルスに感染したフランス人女性への投与について
(58)^ “日本の薬で仏女性快方へ エボラ熱にインフル薬を投与”. スポーツニッポン. (2014年10月3日) 2014年10月4日閲覧。
(59)^ 石︵2018︶p.34
(60)^ 三井美奈 (2014年10月5日). “日本の薬でエボラ出血熱治療、仏の患者退院”. 読売新聞 2014年10月5日閲覧。
(61)^ ﹁アビガン錠200mg﹂のエボラ出血熱患者への投与について
(62)^ “看護助手がエボラを克服、検査で陰性反応 スペイン”. CNN.co.jp. (2014年10月20日) 2014年10月20日閲覧。
(63)^ “Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro”. Manli Wang et al.. Cell Research (Nature) (2020年2月4日). 2020年2月4日閲覧。
(64)^ “对战新冠病毒,多种药物和疗法正在临床发挥效力丨科技战疫进行时”. 张佳星. 科技日报 (2020年2月13日). 2020年2月29日閲覧。
(65)^ “好消息! 磷酸氯喹等部分药物初步显示良好临床疗效”. 科技日报记者 付丽丽 刘垠. 中華人民共和国科学技術部 (2020年2月15日). 2020年2月27日閲覧。
(66)^ 瑞德西韦等药物已初步显示良好临床疗效 ︵中国語︶ 新浪 2020年2月16日
(67)^ ︵钟武、Zhong Wu︶2月4日にCell Researchに発表された論文の共同執筆者の一人
(68)^ “新冠肺炎治疗药物获批上市?别急!注意看这三个字……”. 张佳星. 科技日报 (2020年2月17日). 2020年2月29日閲覧。
(69)^ 全国首个潜在治新冠肺炎药物"法维拉韦"获批上市 ︵中国語︶ 新浪 2020年2月16日
(70)^ the Joint Prevention and Control Mechanism of the State Council
(71)^ 张新民、チャン・シンミン、Zhang Xinmin
(72)^ 上記の深圳第三人民病院における26例は、この投与群35例の一部であると思われる。
(73)^ “科研攻关组药物专班‥法匹拉韦安全性好、疗效明确、药品可及!”. 刘垠. 科技日报 (2020年3月6日). 2020年3月7日閲覧。
(74)^ “科技部‥托珠单抗已用于救治272位重症患者”. 刘垠 刘园园. 科技日报 (2020年3月6日). 2020年3月7日閲覧。
(75)^ ﹁新しいコロナウイルス肺炎︵COVID-19︶患者の治療におけるファピラビルの安全性と有効性に関する臨床研究(登録番号‥ChiCTR2000029600)﹂
(76)^ abc“法匹拉韦最新临床结果显示‥清除新冠病毒平均4天见效且安全性好”. 刘垠. 科普时报(中国科普网) (2020年3月12日). 2020年3月13日閲覧。
(77)^ ab“Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study”. Engineering. (2020-03-18). doi:10.1016/j.eng.2020.03.007.
(78)^ 3月17日付けの科技日報
(79)^ “法匹拉韦显示出很好的临床疗效‥安全性好、疗效明确、药品可及”. 科技日报记者 刘垠 唐芳. 中華人民共和国科学技術部 (2020年3月17日). 2020年3月17日閲覧。
(80)^ ab“新型コロナに﹁アビガン﹂ 軽症者7割、7日以内に回復 中国・武漢大など”. 毎日新聞. 毎日新聞社 (2020年3月24日). 2020年3月25日閲覧。
(81)^ ab“﹁アビガン﹂、7日で7割回復 軽症者に中国チーム”. 47NEWS. 共同通信社 (2020年3月24日). 2020年3月25日閲覧。
(82)^ ︵多中心临床研究、登録番号‥ChiCTR2000030254︶
(83)^ 同研究チームが3月27日に、medRxiVプレプリントサーバ上に投稿した未査読論文による。“Favipiravir versus Arbidol for COVID-19‥ A Randomized Clinical Trial”. medRxiV. (2020-03-20). doi:10.1101/2020.03.17.20037432.
(84)^ “新型インフル薬﹁アビガン﹂活用も、新型肺炎の治療薬に-加藤厚労相”. Bloomberg. (2020年2月2日) 2020年2月2日閲覧。
(85)^ “患者にインフル治療薬﹁アビガン﹂投与 エボラ薬も”. 産経ニュース. 産経新聞社 (2020年2月22日). 2020年2月22日閲覧。
(86)^ “コロナにインフル治療薬﹁アビガン﹂活用を検討”. nikkansports.com. 日刊スポーツ社 (2020年2月22日). 2020年2月22日閲覧。
(87)^ “アビガンの投与開始”. 47NEWS. 共同通信社 (2020年2月22日). 2020年2月22日閲覧。
(88)^ 日本感染症学会ホームページ﹁挿管管理を要した3例を含む新型コロナウイルス感染症15例の報告﹂︵市立札幌病院︶
(89)^ “日本感染症学会 挿管管理を要した3例を含む新型コロナウイルス感染症15例の報告”. 日本感染症学会HP新型コロナウイルス感染症︵COVID-19︶症例報告. (2020-03-19).
(90)^ 向井正也︵札幌市立病院院長︶﹃︻新型コロナ︼感染治療の最前線﹁アビガンが効いていることが多い﹂﹁息が極めて苦しくなる﹂市立札幌病院院長が語る︻HTBニュース︼﹄北海道テレビ放送︵HTB︶、2020年3月26日、該当時間: 1m28s。2020年4月1日閲覧。
(91)^ “新型コロナ治療薬﹁アビガン﹂承認へ 首相表明”. 日本経済新聞. (2020年3月28日) 2020年3月29日閲覧。
(92)^ “富士フイルム、新型コロナに﹁アビガン﹂国内治験開始”. 日本経済新聞 電子版. 日本経済新聞社 (2020年4月1日). 2020年4月1日閲覧。
(93)^ “新型コロナ、富士フイルムがアビガンの治験開始”. www.afpbb.com. フランス通信社 (2020年4月1日). 2020年4月1日閲覧。
(94)^ ab“ファビピラビルを早期投与し軽快した80代後半のCOVID-19 肺炎の1例︵船橋中央病院︶︵2020.3.31︶”. 日本感染症学会HP新型コロナウイルス感染症︵COVID-19︶症例報告. (2020-03-31).
(95)^ 白野倫徳医師︵大阪市立総合医療センター︶﹃︻第2弾︼新型コロナ“感染者治療”行う白野医師に再び聞く…﹃1か月での状況変化﹄﹃日本の医療崩壊の可能性は?﹄﹃人工呼吸器等の増産効果は?﹄﹄毎日放送︵MBS︶、2020年4月2日、該当時間: 14:40。2020年4月13日閲覧。
(96)^ ANNニュース︵2020年4月3日︶,﹁アビガン﹂の原料供給に関するお知らせ~新型コロナウイルス感染拡大防止のため、マロン酸ジエチルを生産~︵2020年4月2日デンカ株式会社︶
(97)^ ab“ファビピラビル︵アビガン®︶投与により速やかな症状改善とPCR陰転化を認めたCOVID-19肺炎︵東京品川病院︶”. 日本感染症学会HP新型コロナウイルス感染症︵COVID-19︶症例報告. (2020-04-06).
(98)^ “i首相﹁医療支援、重症者に﹂ 緊急事態宣言で記者会見”. 日本経済新聞. (2020年4月7日) 2020年4月7日閲覧。
(99)^ “当院におけるCOVID-19診療11例の経験 ―ファビピラビル投与を行った肺炎例を中心に―︵独立行政法人地域医療機能推進機構船橋中央病院︶︵2020.4.13︶”. 日本感染症学会HP新型コロナウイルス感染症︵COVID-19︶症例報告. (2020-04-13).
(100)^ @ソラ豆琴美@skysoy0520 (2020年4月14日). "コロナの症状、アイドル仲間へ、アビガン治療のことを書きました". X︵旧Twitter︶より2020年4月14日閲覧。
(101)^ “学会で治療薬の状況報告 ﹁アビガン﹂などで改善例も”. NHK. (2020年4月18日) 2020年4月18日閲覧。
(102)^ コロナ軽症者向けに﹁宿泊施設21万室を確保﹂ 経財相
(103)^ “厚労相、コロナ治療薬の承認審査﹁できる限り短縮﹂”. 日本経済新聞. (2020年4月22日) 2023年12月23日閲覧。
(104)^ “アビガン投与へ規制緩和を要望”. NHK 福岡. (2020年4月22日) 2020年4月22日閲覧。
(105)^ “コロナウイルス感染症に対するアビガン︵一般名‥ファビピラビル︶に係る観察研究の概要及び同研究に使用するための医薬品の提供について”, 厚生労働省, (2020-04-27) 2020年5月3日閲覧。
(106)^ “安倍首相﹁可能な場合 今月31日待たずに宣言解除﹂ 新型コロナ”. NHK. (2020年5月4日) 2020年5月4日閲覧。
(107)^ “アビガン、福岡で早期投与可能に 軽症者も、医師会発表”. 朝日新聞. (2020年5月11日) 2023年12月23日閲覧。
(108)^ “治療薬アビガン、臨床研究で有効性示せず”. 毎日新聞︵2020年5月20日︶. 2020年6月14日閲覧。
(109)^ “アビガンの審査、治験成績なしでも可 厚労省見解”. 日本経済新聞 電子版︵2020年5月12日︶. 2020年6月14日閲覧。
(110)^ “アビガン、5月中の承認見送りへ 厚労相﹁科学的評価は時期尚早﹂”. 毎日新聞︵2020年5月26日︶. 2020年6月14日閲覧。
(111)^ “アビガン、5月中の承認見送り 厚労相﹁治験を継続﹂”. 日本経済新聞 電子版︵2020年5月26日︶. 2020年6月14日閲覧。
(112)^ “アビガンの5月承認を断念 加藤厚労相﹁治験を継続﹂”. 朝日新聞デジタル︵2020年5月26日︶. 2020年5月26日閲覧。
(113)^ “アビガンの5月承認を断念 効果まだ不明、企業未申請”. 産経新聞. (2020年5月25日) 2020年5月26日閲覧。
(114)^ abcd“︻中間報告︼ファビピラビル観察研究中間報告︵2020年5月15日現在︶”. 日本感染症学会HP新型コロナウイルス感染症︵COVID-19︶への対応について. (2020-05-26).
(115)^ “﹁アビガンで軽症9割回復﹂中間報告 比較検証はできず”. 朝日新聞. (2020年5月26日) 2023年12月23日閲覧。
(116)^ “ファビピラビル(アビガン)特定臨床研究の最終報告について”. 藤田医科大学︵2020年7月10日︶. 2020年7月10日閲覧。
(117)^ ︻中間報告︼ファビピラビル観察研究中間報告︵2020年5月15日現在︶. 一般社団法人 全国医学部長病院長会議. (2020-09-10).
(118)^ “抗インフルエンザウイルス薬﹁アビガン®錠﹂ 新型コロナウイルス感染症患者を対象とした国内臨床第III相試験にて主要評価項目を達成 ︵2020年9月23日︶”. www.fujifilm.com. 2020年9月23日閲覧。
(119)^ “抗インフルエンザウイルス薬﹁アビガン®錠﹂の製造販売承認事項一部変更承認申請 | 富士フイルム”. www.fujifilm.com︵2020年10月16日︶. 2020年10月16日閲覧。
(120)^ “富士フイルム、アビガン販売申請11月にも承認可能性”. 日本経済新聞 電子版︵2020年10月16日︶. 2020年10月16日閲覧。
(121)^ “アビガン、継続審議に 厚労省部会﹁有効性判断は困難﹂”. 朝日新聞デジタル︵2020年12月21日︶. 2020年12月22日閲覧。
(122)^ “アビガン承認見送り、継続審議に 厚労省部会﹁有効性の判断困難﹂”. 毎日新聞. (2020年12月21日) 2020年12月22日閲覧。
(123)^ “富士フイルム、アビガン治験再実施”. 日本経済新聞 (2021年2月21日). 2021年2月25日閲覧。
(124)^ “Fujifilm to restart Avigan COVID-19 trials in Japan” (英語). Nikkei Asia︵2021年2月21日︶. 2021年2月25日閲覧。
(125)^ “抗インフルエンザウイルス薬﹁アビガン®錠﹂新型コロナウイルス感染症患者を対象とした新たな臨床第III相試験を国内で開始”. www.fujifilm.com︵2021年4月21日︶. 2021年4月22日閲覧。
(126)^ アビガン治験、投与終了 重症化率低下で検証困難に - 日本経済新聞 2022年3月11日
(127)^ “アビガンの臨床試験、米国で患者投与へ 富士フイルム‥朝日新聞デジタル”. 朝日新聞デジタル. 2020-04-09. 2020年4月10日閲覧。
(128)^ “First Patient Dosed in Appili Therapeutics’ Phase 3 Clinical Trial of Avigan® Tablets (Favipiravir) for the Treatment of COVID-19 in the United States” (英語). Appili(2020年12月2日). 2021年2月28日閲覧。
(129)^ “Appili Submits Protocol for a Phase 3 Study Evaluating Favipiravir for the Treatment of Patients with COVID-19 Infections to the US FDA” (英語). Appili(2020年9月11日). 2021年2月28日閲覧。
(130)^ ““Appili Therapeutics Provides Update on Phase 3 PRESECO Clinical Trial Evaluating Avigan®/Reeqonus™” (英語).”. Appili. (2021年11月12日). 2021年11月19日閲覧。閲覧。
(131)^ “Japan to Give Iran Free Avigan Drug for Treating Coronavirus”. Tasnim News Agency. (2020年3月20日) 2020年3月27日閲覧。
(132)^ “Iran denies rumors on Japanese drug being effective against coronavirus”. Trend News Agency︵アゼルバイジャン通信社、テヘラン発報道︶. (2020年4月3日) 2020年4月4日閲覧。
(133)^ “Avigan Drug Made in Iran”. Tasnim News Agency (Iran). (2020年4月5日) 2020年4月6日閲覧。
(134)^ “Coronavirus Outbreak in Iran: Death Toll Surpasses 17,500 While Regime Hoards a Possible Cure”. National Council of Resistance of Iran (NCRI). (2020年4月5日) 2020年4月6日閲覧。
(135)^ 2020年3月22日付けのイタリアの日刊紙 Il Fatto Quotidiano の報道など
(136)^ “Coronavirus, il Veneto sperimenta l'antivirale giapponese Favipiravir. Ma l'Aifa: "Ci sono scarse evidenze scientifiche su efficacia"” (イタリア語). Il Fatto Quotidiano (2020年3月22日). 2020年3月23日閲覧。
(137)^ “AIFA precisa, uso favipiravir per COVID-19 non autorizzato in Europa e USA, scarse evidenze scientifiche sull’efficacia” (イタリア語). aifa.gov.it. 2020年3月23日閲覧。
(138)^ 2020年3月24日、米国のアラブ系ニュースサイトAl-Monitor
(139)^ “Turkey says it’s using ‘special’ coronavirus drug sent from China”. Al-Monitor. (2020年3月24日) 2020年3月27日閲覧。
(140)^ “Turkey to locally develop COVID-19 drug, Erdoğan says”. DAILY SABHA. (2020年6月9日) 2020年6月11日閲覧。
(141)^ Riesiger Wirbel um eine Pille gegen CoronaAKTUALISIERT AM 01.04.2020-18:59,FAZ.
(142)^ ドイツ、﹁アビガン﹂大量調達へ 新型コロナ治療に 日本経済新聞2020/4/3 3:24
(143)^ ab“韓国政府、日本のアビガンを新型コロナ治療薬に使用しない方針…﹁臨床的根拠が不十分﹂”. 中央日報 (2020年3月16日). 2020年3月18日閲覧。
(144)^ “UK coronavirus patients set to trial 'promising' Japanese-made drug”. The Evening Standard. (2020年5月1日) 2020年5月2日閲覧。
(145)^ “Coronavirus drug trials that finish next month could see lockdown fully lifted by mid-summer as scientists investigate more than 120 options”. THE DAILY MAIL. (2020年5月2日) 2020年5月2日閲覧。
(146)^ “﹁アビガン﹂のジェネリック、ロシアが新型コロナ薬として暫定承認”. ブルームバーグ. (2020年5月31日) 2020年6月1日閲覧。
(147)^ “Russians claim to have an effective treatment for the coronavirus, which hospitals will start using in June”. CNBC. (2020年6月1日) 2020年6月1日閲覧。
(148)^ “RDIF and ChemRar announce first interim results of clinical trials of Favipiravir drug’s effectiveness in coronavirus therapy”. ChemRar. (2020年5月14日) 2020年5月14日閲覧。
(149)^ “Glenmark Initiates Phase 3 Clinical Trials on Antiviral Favipiravir for COVID-19 Patients in India” (英語). www.prnewswire.com︵2020年5月12日︶. 2020年6月15日閲覧。
(150)^ “COVID-19: Strides Pharma to conduct bio-equivalence study on favipiravir in India” (英語). The Financial Express (2020年5月21日). 2020年6月15日閲覧。
(151)^ “Glenmark to Commence Another New Phase 3 Clinical Trial on a Combination of Two Anti-viral Drugs Favipiravir and Umifenovir in Hospitalized Patients of Moderate COVID-19 in India” (英語). www.prnewswire.com︵2020年5月26日︶. 2020年6月15日閲覧。
(152)^ “Glenmark Becomes the First Pharmaceutical Company in India to Receive Regulatory Approval for Oral Antiviral Favipiravir, for the Treatment of Mild to Moderate COVID-19” (英語). www.prnewswire.com︵2020年6月20日︶. 2020年6月30日閲覧。
(153)^ abcdef“﹁COVID-19 に対する抗ウイルス薬による治療の考え方 第1版﹂の公表について”. 厚生労働省新型コロナウイルス感染症対策推進本部. 2020年9月21日閲覧。
(154)^ abc“COVID-19 に対する抗ウイルス薬による治療の考え方 第1版”, 日本感染症学会, (2020-02-26) 2020年4月8日閲覧。
(155)^ “アビガンの必要処方量”. 2020年9月19日閲覧。
(156)^ “医薬品インタビューフォーム﹁アビガン錠 200mg﹂︵2019年4月改訂︵第4版︶︶”. 富士フイルム株式会社. 2020年9月23日閲覧。
(157)^ abc“ファビピラビルのCOVID-19肺炎患者への使用について 国際感染症緊急事態への国際貢献に係る専門委員会 資料 2020年2月17日 富士フイルム富山化学株式会社”. 2020年9月19日閲覧。
(158)^ “小児における COVID-19 治療薬に対する考え方︵第1版︶”. 日本小児科学会 予防接種・感染症対策委員会. p. 11. 2020年9月21日閲覧。
(159)^ “新型コロナ感染症向けに﹁アビガン﹂増産、9月までに月30万人分生産を実現|Web医事新報|日本医事新報社︵2020年04月25日︶”. 2020年9月22日閲覧。
(160)^ “﹁アビガンで軽症9割回復﹂中間報告 比較検証はできず‥朝日新聞デジタル”. 朝日新聞デジタル. 2020年9月20日閲覧。
(161)^ “富士フイルム、新型コロナに対する﹁アビガン﹂の治験の詳細が明らかに”. 日経バイオテク. 2020年9月24日閲覧。
(162)^ “富士フイルム、10月にもアビガン承認申請”. 日本経済新聞社. 2020年9月24日閲覧。
(163)^ “富士フイルム、10月にもアビガン承認申請”. 日本経済新聞 電子版︵2020年9月23日︶. 2020年9月24日閲覧。
(164)^ “﹁アビガン﹂10月にも承認申請…富士フイルム、コロナ治験で一定の結果 : 医療・健康”. 読売新聞オンライン (2020年9月23日). 2020年9月24日閲覧。
(165)^ “アビガン、年内にも承認の可能性 ﹁早期に症状を改善﹂‥朝日新聞デジタル”. 朝日新聞デジタル︵2020年9月23日︶. 2020年9月24日閲覧。
(166)^ abファビピラビル製剤の使用に当たっての留意事項について - 厚生労働省・2014年3月24日
(167)^ ab“赤江珠緒ラジオにメール アビガン副作用の誤解綴る”. 日刊スポーツ (2020年5月13日). 2023年12月23日閲覧。
(168)^ “新型コロナ治療で目が青色に変わる事例が報告されている”. GIGAZINE (2023年9月5日). 2023年9月5日閲覧。
(169)^ “アビガン錠200mg” (2019年4月). 2020年4月17日閲覧。
(170)^ ﹁新規作用様式のパンデミック対策用抗インフルエンザ薬の開発﹂で平成30年度 科学技術分野の文部科学大臣表彰﹁科学技術賞﹂を受賞2018年4月17日
富山化学工業株式会社
(171)^ 科学技術分野の文部科学大臣表彰文部科学省
参考文献[編集]
- 石弘之『感染症の世界史』KADOKAWA〈角川ソフィア文庫〉、2018年1月(原著2014年)。ISBN 978-4-04-400367-8。
外部リンク[編集]
- 富士フイルム富山化学
- アビガン錠に関する情報
- アビガン錠200㎎ 添付文書 (PDF)
- アビガン錠200mg - 独立行政法人医薬品医療機器総合機構
